In this article
How Band 6 HSC Chemistry Students Study Differently
What are the study habits of band 6 HSC chemistry students?
Studying chemistry in the HSC can be a hard and strenuous process but it doesn’t have to be. In this article, we share the habits which differentiate band 6 HSC chemistry students from their peers.
Typically, band 6 HSC Chemistry students:
- have a solid understanding of Year 11 Chemistry
- strive to gain extensive understanding of key concepts
- invest time into studying practical investigations
- make rigorous checklists to manage their studies
- incorporate practice exams into their studies regularly
- use marking guidelines to improve their ability to answer extended response questions
1. Band 6 HSC Chemistry students have solid understanding of Year 11 Chemistry
The HSC chemistry course builds upon the concepts of Year 11 Chemistry. To cope with the demand of Year 12 Chemistry course, students need to be equipped with a thorough understanding of:
- basic mole calculations,
- gas laws, bonding,
- collision theory
- and entropy and enthalpy.
High achieving chemistry students have an in-depth knowledge and understanding of Year 11 chemistry concepts.
Students with a mastery of Year 11 Chemistry will be able to quickly learn the harder Year 12 Chemistry concepts, saving a significant amount of time in the fast paced marathon that is the HSC.
For example, if you have a comprehensive understanding of molarity and concentrations, you will find most of the titration calculation quite easy and straightforward while other students may struggle.
Consider the following typical titration question:
Year 12 Chemistry Question on Titration
| Question Potassium hydroxide was titrated against 25.00mL aliquots of 0.12M sulfuric acid. The average volume of potassium hydroxide used was 39.85mL. Calculate the concentration of the base. (3 marks) |
| Worked solution Step 1: As with any calculation in chemistry, the first step is to write a balanced chemical equation H2SO4(aq) + 2KOH(aq) → K2SO4(aq) + 2H2O(l) Step 2: From the information provided in the question we can calculate the number of moles of sulfuric acid using n = c × v n(H2SO4) = 0.12 × 0.025 = 0.003 Step 3: Using stoichiometry, we can determine the number of moles of potassium hydroxide required to neutralise the sulfuric acid n(KOH) = n(H2SO4) × 2 = 0.003 × 2 = 0.006 Step 4: Now that we have the volume and number of moles of KOH, we can use c = \frac{n}{v} , to determine the concentration of potassium hydroxide c(KOH) = \frac {n(KOH)}{V} = \frac {0.12\times0.025\times2}{0.03985} = 0.15 M (2 s.f.) |
As you can see, aside from some new terminology, there is nothing particularly new or challenging about this calculation, provided you have mastered your year 11 content.
Similarly, much of the physical properties of organic molecules studied in module 7 will only seem like a small extension of what you already know if you have a strong understanding of chemical bonding and intermolecular forces from year 11.
Key year 11 chemistry concepts you should know for year 12
The following table lists the key year 11 chemistry concepts that you need to gain mastery of in order to ace each module in year 12.
| Year 12 Chemistry Modules | Year 11 Chemistry Concepts |
| Module 5: Equilibrium and Acid Reactions | Module 3: Reactive Chemistry Collision Theory Rates of Reactions Module 4: Drivers of Reactions Enthalpy Entropy and Gibbs Free Energy |
| Module 6: Acid/Base Reactions | Module 2: Introduction to Quantitative Chemistry Mole concept Concentration and molarity Module 3: Reactive Chemistry Chemical Reactions |
| Module 7: Organic Chemistry | Module 1: Properties and Structures of Matter Bonding Intermolecular and Intramolecular forces and how they relate to physical properties Module 3: Reactive Chemistry Oxidation reactions Module 4: Drivers of Reactions Enthalpy and calorimetry |
| Module 8: Applying Chemical Ideas | Module 1: Properties and Structures of Matter Calculating empirical and molecular formulae from percentage composition Flame test |
Actionable advice #1
Set aside some time and revise these concepts before moving on to studying harder ones.
In the same way that year 12 chemistry relies on year 11 chemistry, each consecutive module relies on the knowledge and skills you have gained from the previous one.
Make sure to seek clarification consistently as you learn, otherwise, it will have a snowballing effect.
For those in year 11, make sure to take your studies seriously. Even though your final grades won’t contribute to your HSC, what you learn will lay the necessary groundwork for your studies ahead.
2. Band 6 HSC Chemistry students strive to gain extensive understanding of key concepts
Students who achieve a band 6 in HSC Chemistry generally have a genuine interest for the subject. They refrain from the temptation to simply study the content outlined in the syllabus but seek to deepen their understanding by conducting further research.
Whenever they come across a concept they do not fully understand or which seems incomplete, instead of accepting them as facts and growing complacent, they question their teachers and scour the internet until they have a comprehensive understanding of the topic.
In spending time to fully understand a concept and the cause and effect relationship which exists for various concepts, it eliminates the need for memorising facts.
Having a deeper conceptual understanding beyond the scope of the syllabus, will allow students to provide a more in depth explanation of concepts and tackle the difficult questions.
These questions, which aim to differentiate between band 5 and 6 students will test depth of understanding and usually address concepts in a creative and different perspective. Therefore, it is essential to have a holistic and complete understanding of the concepts.
Sample Student Responses
Consider the following extended responses from two different students.
Student A: Band 3 response 2/6

As you can see, student A is unable elaborate on his ideas and explain the cause and effect relationship between altering reaction conditions and shifts in equilibrium. This reflects his attempt of rote learning the content rather than developing an in-depth understanding of the concepts.
Student B: Band 6 Response – 6/6


On the other hand, student B has a comprehensive understanding of chemical synthesis and design. This allows her to clearly articulate her ideas in a concise and structured manner and earn full marks.
Actionable advice #2
Rather than rote-learning, work towards developing a clear and extensive knowledge and understanding of key concepts. This will remove the need for memorising facts.
3. Band 6 HSC Chemistry students invest time into studying practical investigations
One of the most common mistakes students make is to not invest time into studying chemical practicals.
60% of the school assessments are allocated for examining Working Scientifically Skills. Practical based questions appear in HSC exams.
Therefore, it is important that you understand exactly what happens during the experiment, why things are done in a certain way and any observations made during the procedure.
How to Study Chemistry Practical Investigations
The most successful chemistry students keep on top of all the chemistry practicals.
One strategy of doing this is to write up brief practical reports every time you complete a practical at school. Include things such as:
- The equipment used, reasons for their use and a diagram of the experimental set up
- Risk assessment
- A detailed method for the experiment
- Observations and/or results of the experiment
In addition to this, make sure to review the essential working scientifically skills which are often assessed in both practical and knowledge based exams.
What are the chemistry practical investigations you must be familiar with?
The mandatory chemistry practicals for the HSC Chemistry Course are outlined below
| Year 12 Chemistry Module | Topics and their Practical Investigations |
| 5. Equilibrium and Acid Reactions | Static and Dynamic Equilibrium
Calculating the Equilibrium Constant Keq
Solution Equilibria
|
| 6. Acid/Base Reactions | Properties of Acids and Bases
Using Brønsted Lowry Theory
Quantitative Analysis
|
| 7. Organic Chemistry | Hydrocarbons
Alcohols
|
| 8. Applying Chemical Ideas | Analysis of Inorganic Substances
Analysis of Organic Substances
|
Source: NSW Educations Standard Authority
Actionable advice #3
Be familiar with all the mandatory practicals as they represent a large percentage of your overall school assessments and is assessed in the HSC Chemistry exams.
4. Band 6 HSC Chemistry students make rigorous checklists to manage their studies
Poor performing students fail to pinpoint their areas for improvement and go around complaining, ‘I don’t know what I don’t know’.
However, band 6 HSC chemistry students have a structured approach to their learning and have strategies to quickly identify their weaknesses.
The structured nature of chemistry means that it is relatively easy to break down the syllabus to identify areas of improvement.
They carefully review the syllabus and make checklists of what they should be able to do after completing each module.
A checklist of organic chemical reactions from Module 7: Organic Chemistry used by a band 6 chemistry student is shown below.

Another way to keep track of your understanding of a topic is through the Learnable quiz performance dashboard. It provides an overview of your performance in each specific topic allowing you to instantly identify any weaknesses.
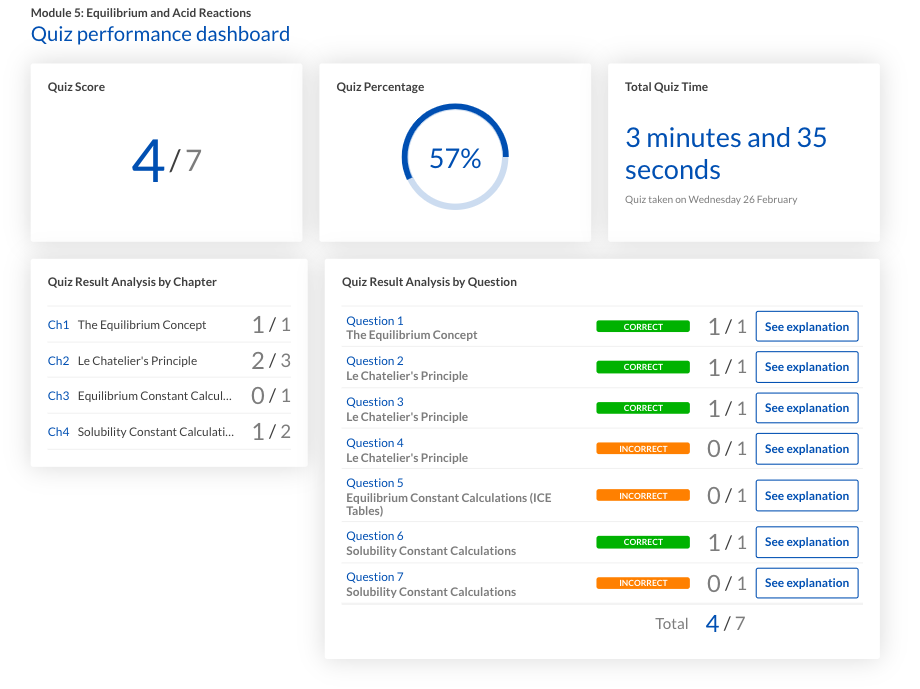
Exams can be broken down too!
After trial exams, it might be overwhelming and difficult to determine what to study next. But just as the chemistry syllabus can be broken down, so can exams. Chemistry exams can be generally broken down to:
- Content: Do you know your content for every module?
- Extended response questions: Can you structure and answer extended response questions in a clear and concise manner?
- Calculation Questions: If you made any mistakes in your calculation questions, is it because of a silly mistake or do you need to revise how to do that particular style of question?
- First hand investigations: Can you recall your first hand investigation and draw relevant diagrams?
Actionable advice #4
Install a system for identifying and addressing your areas for improvement. Whether it is via a simple checklist or a sophisticated software, it is important to know your strengths and weaknesses for each module.
5. Band 6 HSC Chemistry students incorporate exam practice into their studies
While most students save practice papers to do a week before their test, high achieving students expose themselves to a variety of exam style questions every time they learn a new concept.
To maximise their results, band 6 HSC chemistry students spend time fine-tuning their exam skills through regular exam practices.
In doing so, they become more familiar with the formatting of exam questions and the content commonly tested. By undertaking practice exams under timed conditions, they also develop many essential skills such as:
- Learning to work under time pressure
- Finding the balance between speed and accuracy
- Unpacking, planning and structuring extended response questions
How to Incorporate Exam Practice into your Studies
Step 1: Once you have studied a key concept in a particular module, compile any relevant examination questions you can find on that concept. Sources of exam style questions include
- Past HSC Chemistry Papers
- NESA Additional Sample Chemistry Questions
- School Trial Papers
- Past VCE Chemistry Papers
For more practice questions, read 20 Must Know HSC Chemistry Questions
Step 2: Calculate the time required to complete the questions by adding up the marks and multiplying it by 1.5 minutes. In the HSC Chemistry exam, you will have approximately 1.8 minutes per mark or 100 marks of exam questions to complete in 3 hours.
Step 3: Set up a timer and complete the exam style questions within the allocated time. Make sure to do this in a quiet environment that reflects the exam conditions as much as possible. Some students even like to do these practice exams in the same place that they will be taking their test.
Step 4: Mark the questions using the provided marking criteria and note down any mistakes and areas for improvement. Record your mark for this exam in a notebook or on your laptop. This will allow you to come back and reflect on your progress in each topic as you complete more practice exams.
Try to gain exam practice and exposure to exam style questions consistently throughout your studies. The short customisable quizzes on Learnable are a great tool for specific and targeted practice on exam style questions.
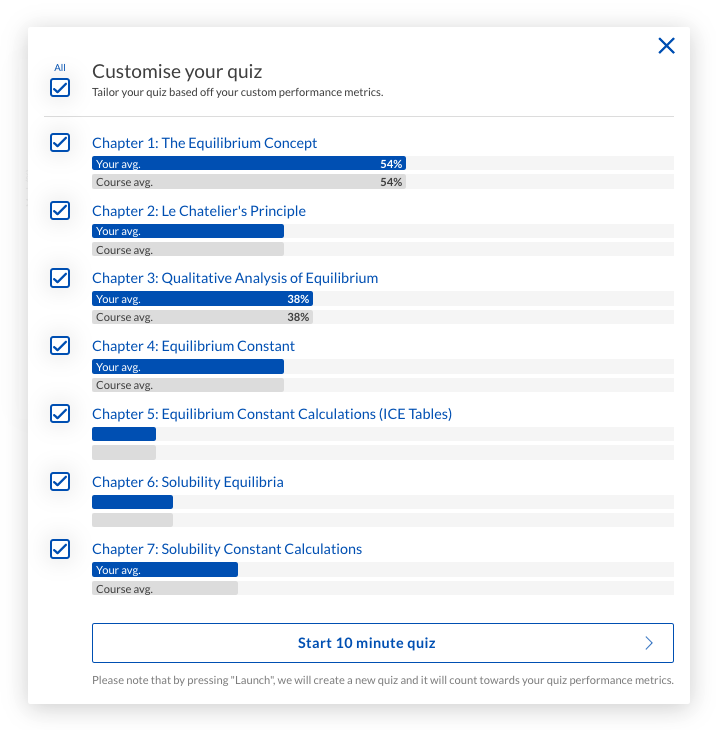
They are perfect for getting some additional practice in if you are on the go and don’t have time to follow the procedure outlined above.
Actionable advice #5
Do past HSC exam papers regularly and become familiar with the format, style and difficulty of the HSC exam questions.
6. Band 6 HSC students use the HSC Chemistry marking guidelines to improve their ability to answer extended response questions
Even though you have mastered all the concepts and calculations, gaining marks in exams and assessment tasks is another game in itself.
A few missing key words in your responses could cost you vital marks.
Therefore, it is important to find out what examiners are looking for in exams.
Band 6 students do this by paying attention to school trial papers and and marking guidelines. From analysing the marking criteria, they are able to determine common mistakes and what is necessary in gaining full marks in responses.
The HSC and VCE chemistry examination report is a great tool in highlighting the common traps and pitfalls other students make and are explicit in stating what is required for a full mark response.
The following is a sample question and marking guideline taken from the 2019 NESA Chemistry Additional Sample Examination Questions
Sample Question
The graph shows changes in pH for the titrations of equal volumes of solutions of two monoprotic acids, Acid 1 and Acid 2
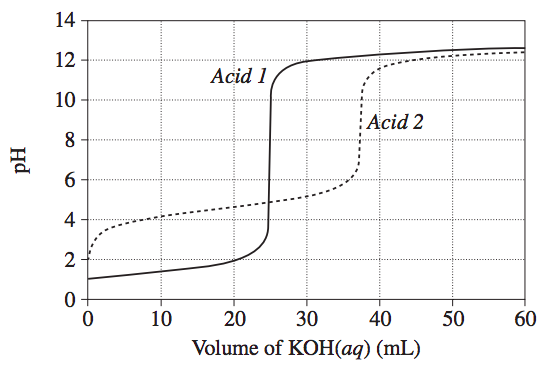
| Explain the differences between Acid 1 and Acid 2 in terms of their relative strengths and concentrations. | 3 |
Marking Guidelines
| Criteria | Marks |
| 3 |
OR
| 2 |
| 1 |
How to use marking guidelines to write band 6 responses
| Step | Advice | Example |
| 1 a)
| Read the marking guidelines and identify the key concepts you need to demonstrate your understanding in. | From the example above, these key concepts include:
|
| 1 b) | For each concept, allocate appropriate marks to determine the depth of the required response. | Since each of the concepts are worth 1 mark, spend no more than 2 to 3 sentences on each. |
| 2 | Relate the key concepts identified by introducing the relevant scientific terms and equations. | Key terms you may consider including:
|
| 3 | Use the key verb in the question or the marking guidelines to determine the structure of your response. For an explain question, Learnable recommends the use of CEO Framework ™ Cause Effect Outcome | Notice for 2 marks, students only need to ‘describe’ the strengths and concentrations of the acid. However, for full marks, students must ‘relate’ these concepts to the pH of equivalence point and the amount of base used. |
Actionable advice #6
Use marking guidelines to develop an understanding of the criteria for gaining full marks in extended response questions.
Learnable Education and www.learnable.education, 2019. Unauthorised use and/or duplications of this material without express and written permission from this site's author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Learnable Education and www.learnable.education with appropriate and specific direction to the original content.