What is the HSC Chemistry Formula and Data Sheet?
All students are provided with the Chemistry Formula and Data Sheet in the HSC Chemistry Exam.
The HSC Chemistry formula sheet contains formulas of key Chemistry concepts and frequently used constants that are taught in the Year 11 & 12 Chemistry course.
The HSC Chemistry Data Sheet consists of
HSC Chemistry Formulae Sheet
| n = \dfrac{m}{MM} | c=\dfrac{n}{V} |
| q=mc \Delta T | \Delta G\degree = \Delta H \degree - T\Delta S\degree |
| pK_a=-log_{10}[K_a] | A=\epsilon lc = log_{10}\dfrac{I_0}{I} |
| PV=nRT | pH = -log_{10}[H^+] |
| Avogadro’s constant, NA | 6.022 × 1023 mol-1 |
| Volume of 1 mole of ideal gas: at 100kPa and | |
| at 0˚C (273.15K) | 22.71 L |
| at 25˚C (298.15K) | 24.79 L |
| Gas constant | 8.314 J mol-1 K-1 |
| Ionisation constant for water at 25˚C (298.15K), Kw | 1.0 × 10-14 |
| Specific heat capacity of water | 4.18 × 103 J kg-1 K-1 |
HSC Chemistry Data Sheet
Solubility constants at 25˚C
| Compound | Ksp | | Compound | Ksp |
| Barium carbonate | 2.58×10-9 | | Lead(II) bromide | 6.60×10-6 |
| Barium hydroxide | 2.55×10-4 | | Lead(II) chloride | 1.70×10-5 |
| Barium phosphate | 1.3×10-29 | | Lead(II) iodide | 9.8×10-9 |
| Barium sulfate | 1.08×10-10 | | Lead(II) carbonate | 7.40×10-14 |
| Calcium carbonate | 3.36×10-9 | | Lead(II) hydroxide | 1.43×10-15 |
| Calcium hydroxide | 5.02×10-6 | | Lead(II) phosphate | 8.0×10-43 |
| Calcium phosphate | 2.07×10-29 | | Lead(II) sulfate | 2.53×10-8 |
| Calcium sulfate | 4.93×10-5 | | Magnesium carbonate | 6.82×10-6 |
| Copper(II) carbonate | 1.4×10-10 | | Magnesium hydroxide | 5.61×10-12 |
| Copper(II) hydroxide | 2.2×10-20 | | Magnesium phosphate | 1.04×10-24 |
| Copper(II) phosphate | 1.40×10-37 | | Silver bromide | 5.35×10-13 |
| Iron(II) carbonate | 3.13×10-11 | | Silver chloride | 1.77×10-10 |
| Iron(II) hydroxide | 4.87×10-17 | | Silver carbonate | 8.46×10-12 |
| Iron(III) hydroxide | 2.79×10-39 | | Silver hydroxide | 2.0 ×10-8 |
| Iron(III) phosphate | 9.91×10-16 | | Silver iodide | 8.52×10-17 |
| | | Silver phosphate | 8.89×10-17 |
| | | Silver sulfate | 1.20×10-5 |
Infrared absorption data
| Bond | Wavenumber / cm-1 |
N—H
(amines) | 3300 – 3500 |
O—H
(alcohols) | 3230 – 3550
(broad) |
| C—H | 2850 – 3300 |
O—H
(acids) | 2500 – 3000
(very broad) |
| C\equivN | 2220 – 2260 |
| C=O | 1680 – 1750 |
| C=C | 1620 – 1680 |
| C—O | 1000 – 1300 |
| C—C | 750 – 1100 |
13C NMR chemical data shift
| Type of carbon | \delta/ppm |
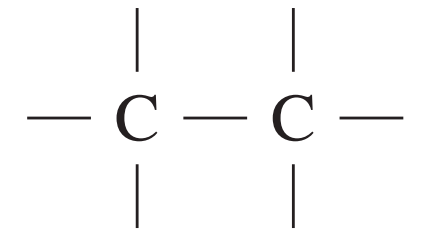 | 5 – 40 |
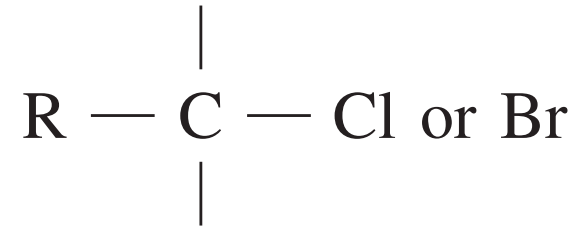 | 10 – 70 |
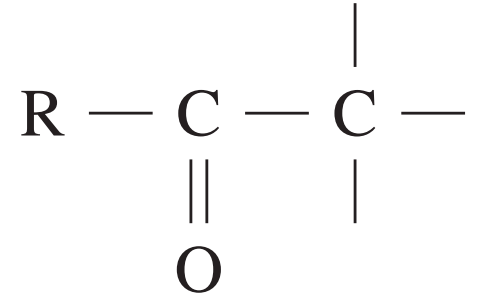 | 20 – 50 |
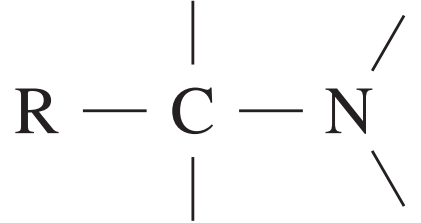 | 25 – 60 |
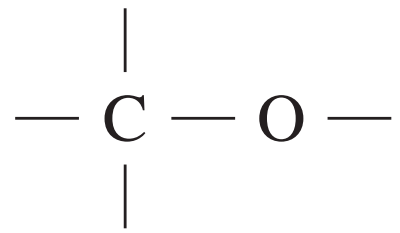
alcohols, ethers or esters | 50 – 90 |
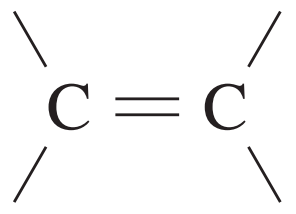 | 90 – 150 |
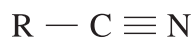 | 110 – 125 |
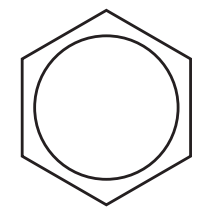 | 110 – 160 |
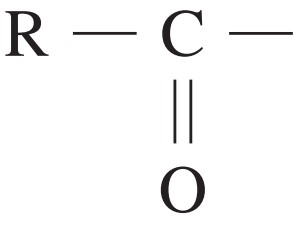
esters or acids | 160 – 185 |
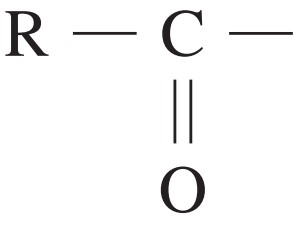
aldehydes or ketones | 190 – 220 |
UV absorption
(This is not a definitive list and is approximate.)
| Chromophore | \lambda_{max} (nm) |
| C—H | 122 |
| C—C | 135 |
| C=C | 162 |
| C\equivC | 173 178
196 222 |
| C—Cl | 173 |
| C—Br | 208 |
Some standard potentials
| K+ + e‾ | \leftrightharpoons | K(s) | -2.94 V |
| Ba2+ + 2e‾ | \leftrightharpoons | Ba(s) | -2.91 V |
| Ca2+ + 2e‾ | \leftrightharpoons | Ca(s) | -2.87 V |
| Na+ + e‾ | \leftrightharpoons | Na(s) | -2.71 V |
| Mg2+ + 2e‾ | \leftrightharpoons | Mg(s) | -2.36 V |
| Al3+ + 3e‾ | \leftrightharpoons | Al(s) | -1.68 V |
| Mn2+ + 2e‾ | \leftrightharpoons | Mn(s) | -1.18 V |
| H2O + e‾ | \leftrightharpoons | \dfrac{1}{2} H2(g) + OH‾ | -0.83 V |
| Zn2+ + 2e‾ | \leftrightharpoons | Zn(s) | -0.76 V |
| Fe2+ + 2e‾ | \leftrightharpoons | Fe(s) | -0.44 V |
| Ni2+ + 2e‾ | \leftrightharpoons | Ni(s) | -0.24 V |
| Sn2+ + 2e‾ | \leftrightharpoons | Sn(s) | -0.14 V |
| Pb2+ + 2e‾ | \leftrightharpoons | Pb(s) | -0.13 V |
| H+ + e‾ | \leftrightharpoons | \dfrac{1}{2} H2(g) | 0.00 V |
| SO42- + 4H+ + 2e‾ | \leftrightharpoons | SO2(aq) + 2H2O | 0.16 V |
| Cu2+ + 2e‾ | \leftrightharpoons | Cu(s) | 0.34 V |
| \dfrac{1}{2}O2(g) + H2O + 2e‾ | \leftrightharpoons | 2OH‾ | 0.40 V |
| Cu+ + e‾ | \leftrightharpoons | Cu(s) | 0.52 V |
| \dfrac{1}{2}I2(s)+ e‾ | \leftrightharpoons | I‾ | 0.54 V |
| \dfrac{1}{2}I2(aq) + e‾ | \leftrightharpoons | I‾ | 0.62 V |
| Fe3+ + e‾ | \leftrightharpoons | Fe2+ | 0.77 V |
| Ag+ + e‾ | \leftrightharpoons | Ag(s) | 0.80 V |
| \dfrac{1}{2}Br(l) + e‾ | \leftrightharpoons | Br‾ | 1.08 V |
| \dfrac{1}{2}Br2(aq) + e‾ | \leftrightharpoons | Br‾ | 1.10 V |
| \dfrac{1}{2}O2(g) + 2H++ 2e‾ | \leftrightharpoons | H2O | 1.23 V |
| \dfrac{1}{2}Cl2(g)+e‾ | \leftrightharpoons | Cl‾ | 1.36 V |
| \dfrac{1}{2}Cr2O72‾+ 7H+ + 3e‾ | \leftrightharpoons | Cr3+ + \dfrac{7}{2} H2O | 1.36 V |
| \dfrac{1}{2}Cl2(aq) + e‾ | \leftrightharpoons | Cl‾ | 1.40 V |
| MnO4– + 8H+ + 5e‾ | \leftrightharpoons | Mn2+ + 4H2O | 1.51 V |
| \dfrac{1}{2} F2(g) + e‾ | \leftrightharpoons | F‾ | 2.89 V |
Source: NSW Education Standards Authority
Written by Hee-Chan Jang
Hee-Chan is the author of Chemistry resources on Learnable. He loves teaching and helping students to "learn smarter", using his multidisciplinary knowledge of science and engineering. He is also currently completing his doctoral degree in mineral processing at The University of Sydney.
Learnable Education and www.learnable.education, 2019. Unauthorised use and/or duplications of this material without express and written permission from this site's author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Learnable Education and www.learnable.education with appropriate and specific direction to the original content.