In this article
Organic Chemistry Reactions Cheatsheet | HSC Chemistry
Read the complete list of the 15 organic chemistry reactions year 12 Chemistry students must know for the HSC. Ace your HSC Chemistry exam with this free cheatsheet.
Organic Chemistry Reactions
Keeping track of organic chemistry reactions in Year 12 Chemistry can be overwhelming. Here is a comprehensive list of all the organic chemistry reactions you should know before your HSC Chemistry exam.
In this article, we cover
- Addition reactions
- Substitution reactions
- Elimination
- Hydrolysis
- Oxidation
- Condensation
- Other reactions
- Practice reaction pathway questions
Addition Reactions
Addition reactions are organic reactions that occur when atoms are added across the double bond of an unsaturated molecule.
Hydrogenation
alkene + hydrogen gas → alkane
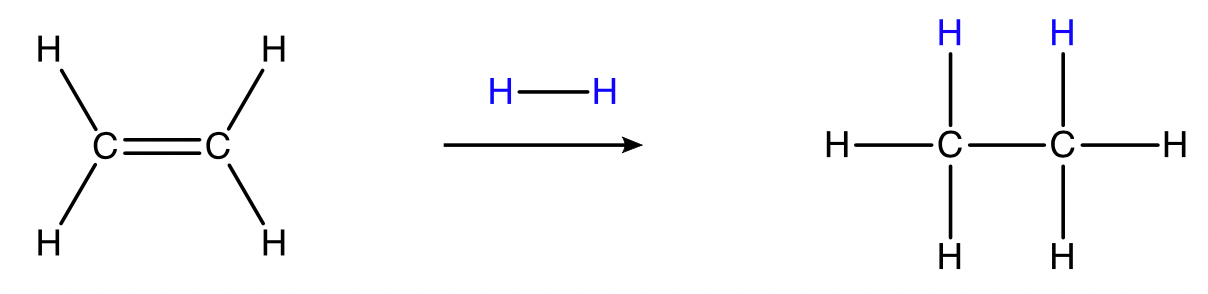
Conditions: \small H_{2(g)} , metal catalyst (such as \small Pt , \small Pd , \small Ni, \small Rh ), room temperature.
Halogenation (bromination and chlorination)
alkene + bromine/chlorine → haloalkane
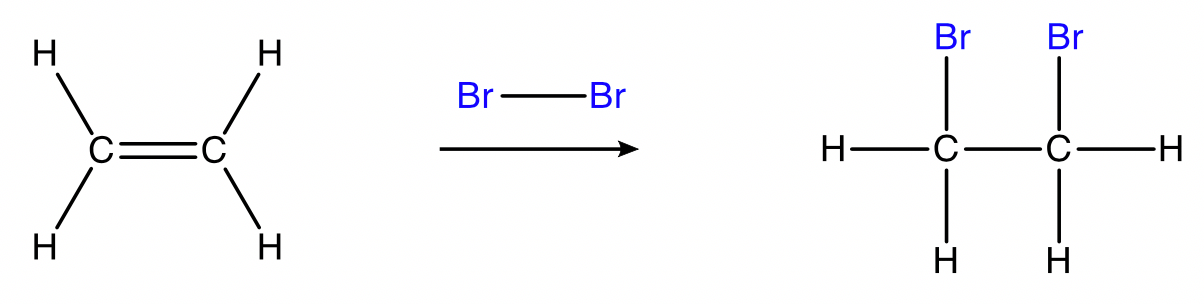
Conditions: \small Br_{2(l) \text{ or } (aq)} or \small Cl_{2(l) \text{ or } (aq)}, room temperature
Hydrohalogenation
alkene + hydrogen halide → haloalkane
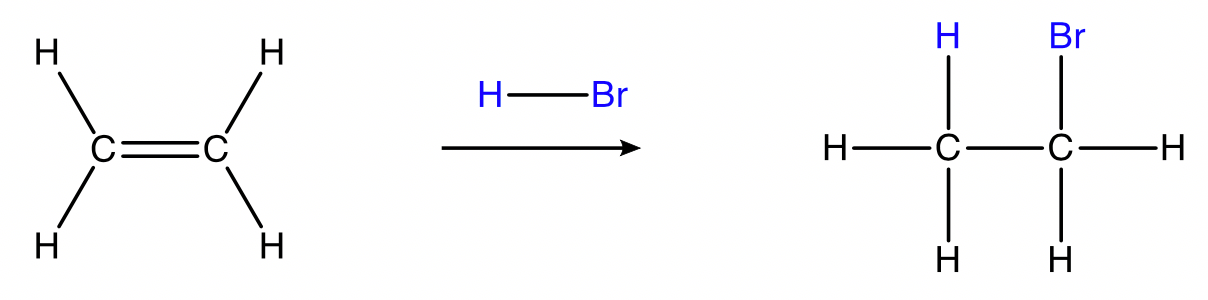
Conditions: \small HF_{(g)} , \small HCl_{(g)} , \small HBr_{(g)} or \small HI_{(g)}, room temperature
Hydration of alkenes
alkene + water → alcohol
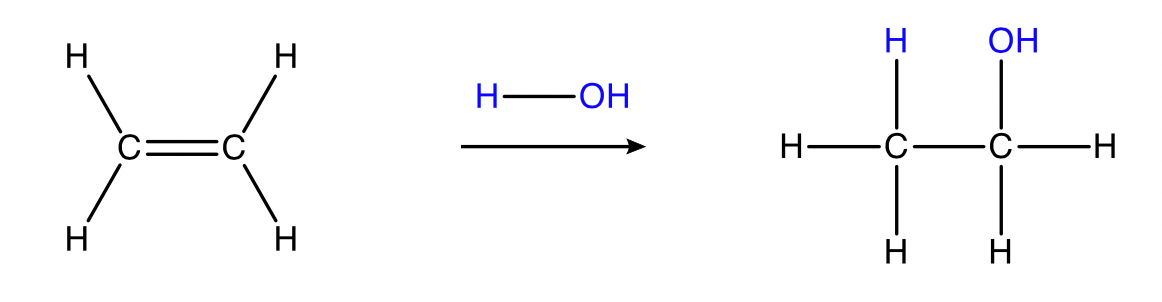
Conditions: \small H_2O , dilute aqueous acid catalyst (e.g. dilute \small H_2SO_{4(aq)} ), heat
Substitution Reactions
A substitution reaction is an organic reaction that occurs when an atom or functional group in a molecule is replaced or substituted by another atom or group.
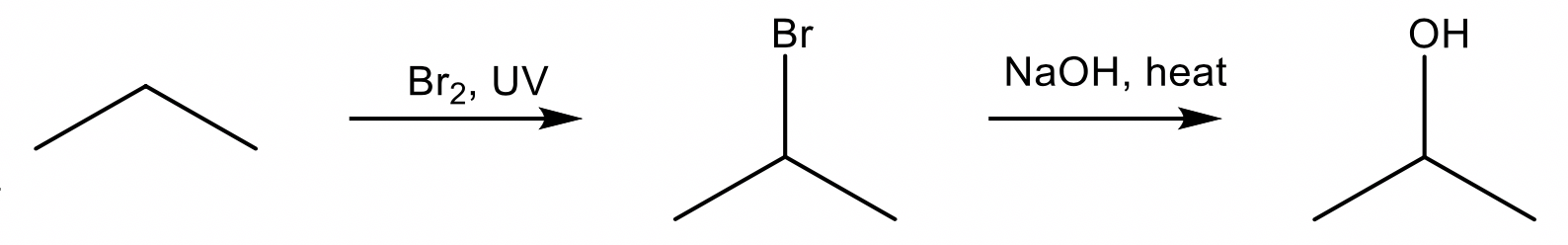
Substitution of alkanes
alkane + halogen → haloalkane + hydrogen halide
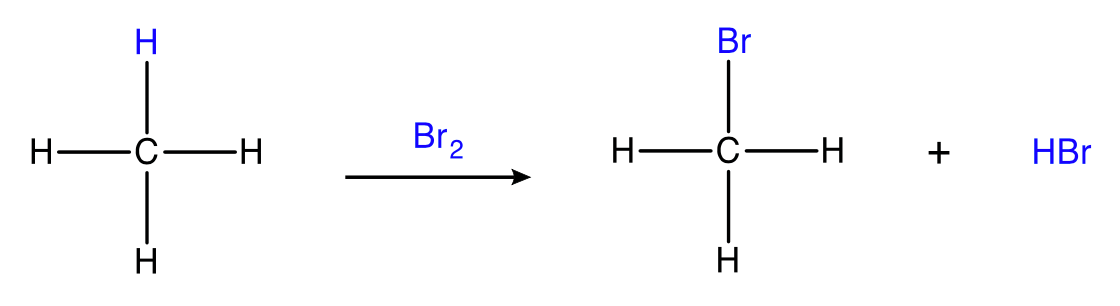
Conditions: \small Br_2 or \small Cl_2 , UV radiation
Substitution of alkanes can only be carried out with chlorine or bromine as fluorine is too reactive (reacts explosively) and iodine is too unreactive.
Substitution of alcohols
alcohol + hydrogen halide → haloalkane + water
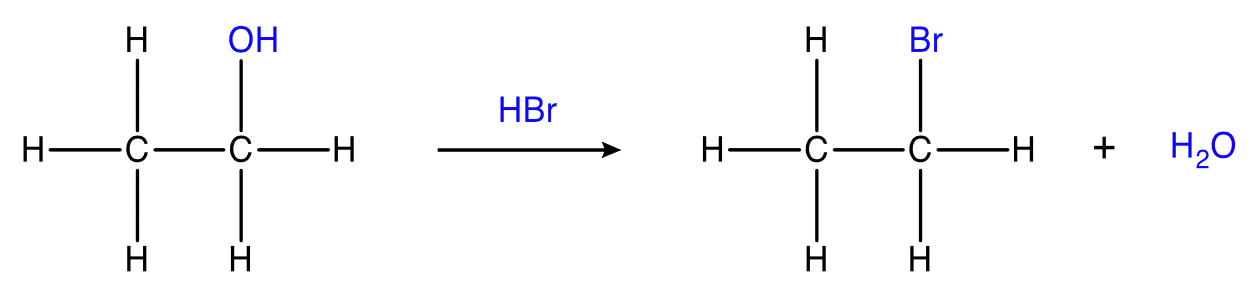
Conditions: \small HCl , \small HBr , \small HI . Tertiary alcohols (3 \small \degree ) react rapidly with \small HX at room temperature while secondary (2 \small \degree ) and primary (1 \small \degree ) alcohols require higher temperatures.
Substitution of haloalkanes
haloalkane + sodium/potassium hydroxide → alcohol + metal halide
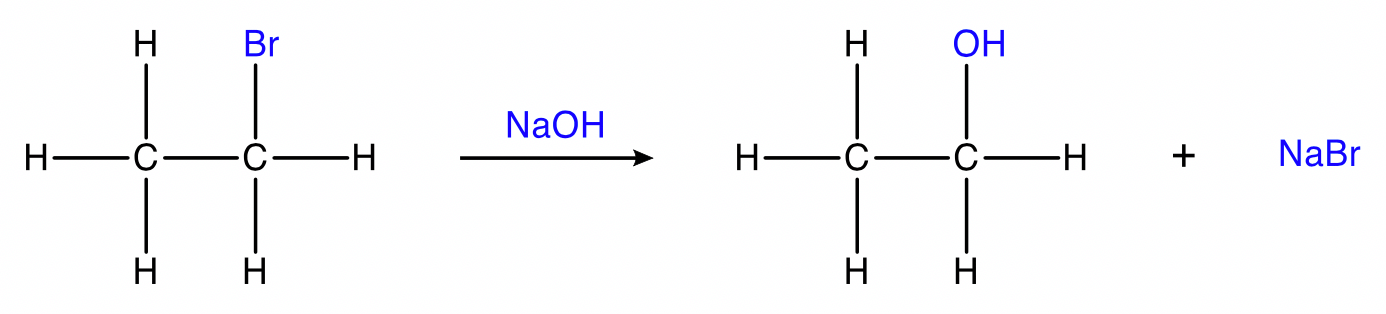
Conditions: \small NaOH_{(aq)} , heat. Fluoroalkanes do not undergo substitution.
Elimination Reactions
An elimination reaction occurs when a single reactant splits into two products. In a sense, it is opposite of addition reactions.
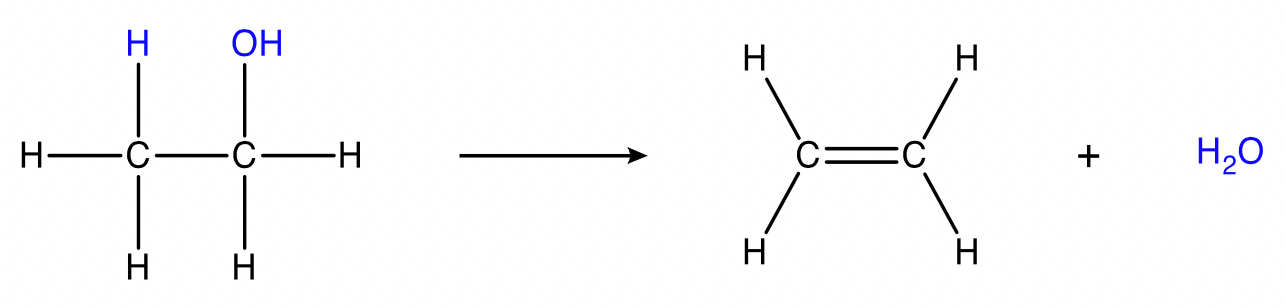
Dehydration
alcohol → alkene + water
Conditions: Concentrated acid catalyst (e.g concentrated \small H_2SO_4 or \small H_3PO_4 ) or \small Al_2O_3 catalyst, heat.
Tertiary alcohols react readily at room temperature while primary and secondary alcohols require very high temperatures.
This reaction can be performed only on alcohols that have at least one hydrogen on the adjacent carbon. For example, methanol cannot undergo dehydration.
Hydrolysis Reactions
A hydrolysis reaction is an organic chemical reaction which involves the breaking of a chemical bond by the addition of water.
Saponification
ester + sodium hydroxide → sodium carboxylate + alcohol
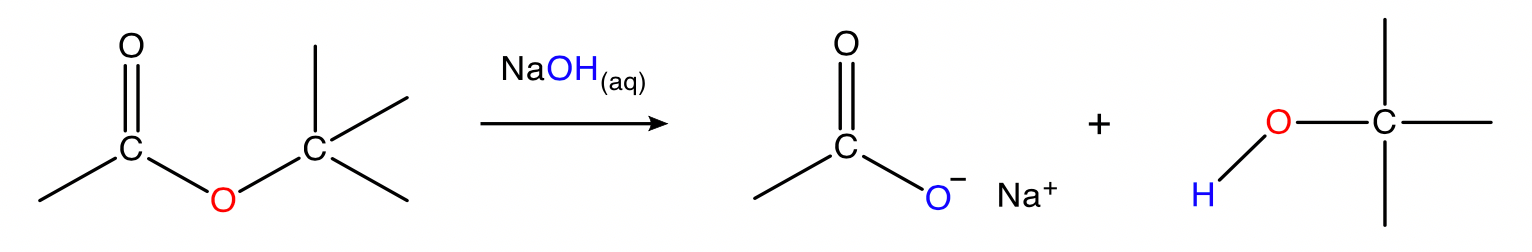
Conditions: \small NaOH_{(aq)} , heat.
Oxidation Reactions
Oxidation reactions involve a transfer of electrons from one reactant to another. In organic chemistry, oxidation refers to loss or gain of electrons by carbon.
Oxidation of alcohols
The products of alcohol oxidation depend on the type of alcohol reacted and the reaction conditions.
Primary alcohols
Primary alcohols are oxidised stepwise to first produce aldehydes then carboxylic acids.
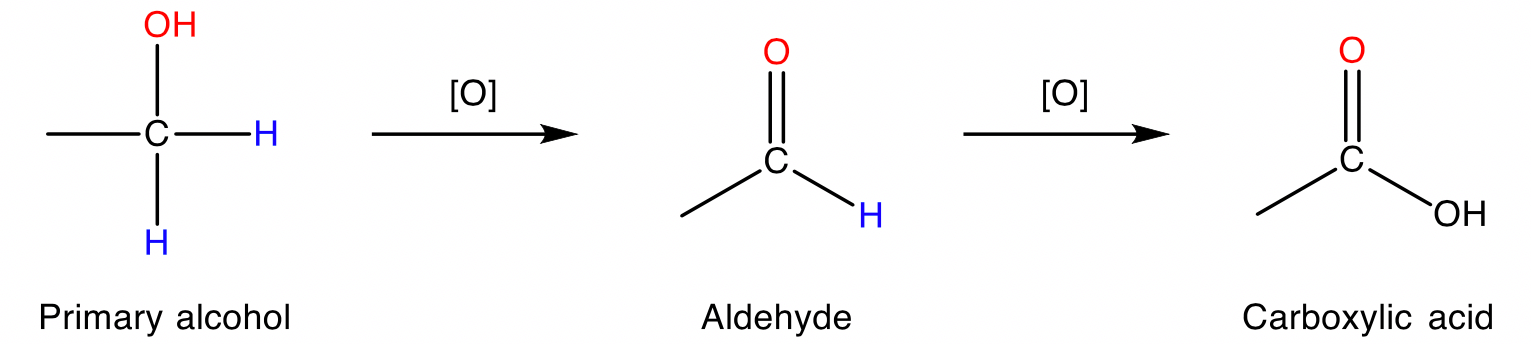
Conditions: Acidified permanganate ion \small ({MnO_4}^-) or dichromate ion \small ({Cr_2O_7}^{-2}) , heat.
To produce aldehydes in a controlled oxidation, milder oxidising agents like pyridinium chlorochromate (PCC) can be used.
Secondary alcohols
Secondary alcohols are oxidised to produce ketones.
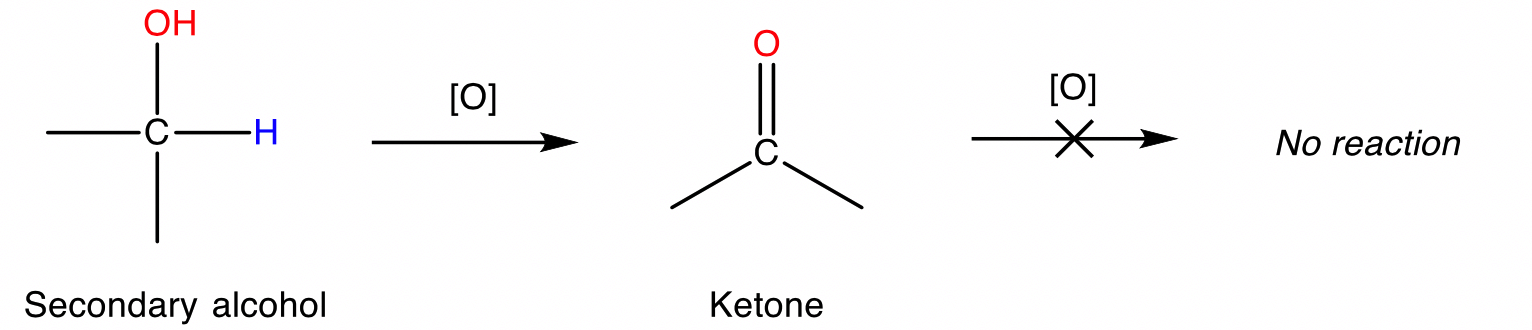
Conditions: Acidified permanganate ion \small ({MnO_4}^-) or dichromate ion \small ({Cr_2O_7}^{-2}) , heat.
Tertiary alcohols
Tertiary alcohols cannot be oxidised.
Combustion of hydrocarbons and alcohols
Complete combustion
fuel + oxygen → carbon dioxide + water
Fuel_{(s/l/g)} + O_{2(g)} \rightarrow CO_{2(g)} + H_2O_{(l)}
Incomplete combustion
fuel + oxygen → carbon soot + carbon monoxide + water
Fuel_{(s/l/g)} + O_{2(g)} \rightarrow C_{(s)} + CO_{(g)} + H_2O_{(l)}
Condensation Reactions
A condensation reaction occurs when two or more molecules combine to form a larger molecule, with the simultaneous elimination of a small molecule such as water or methanol.
Condensation of carboxylic acid and amine
carboxylic acid + amine \rightleftharpoons amide + water
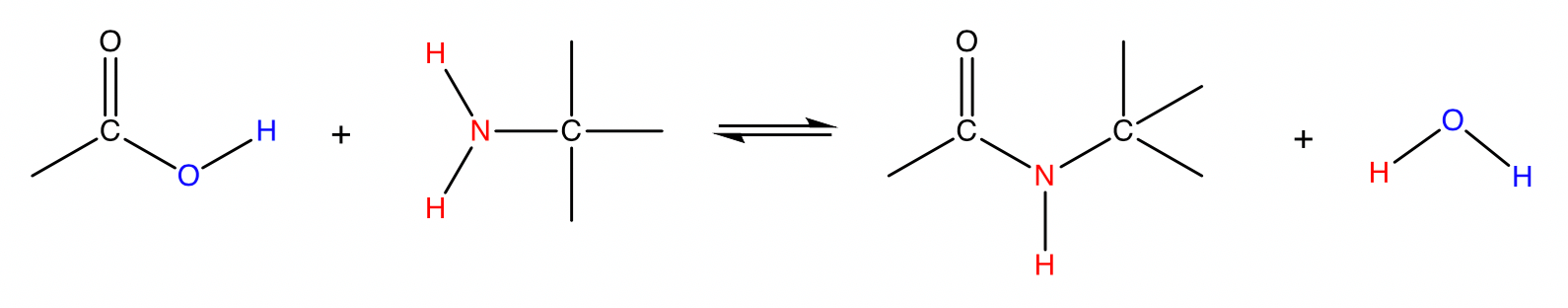 Conditions: Heat
Conditions: Heat
Esterification
carboxylic acid + alcohol → ester + water
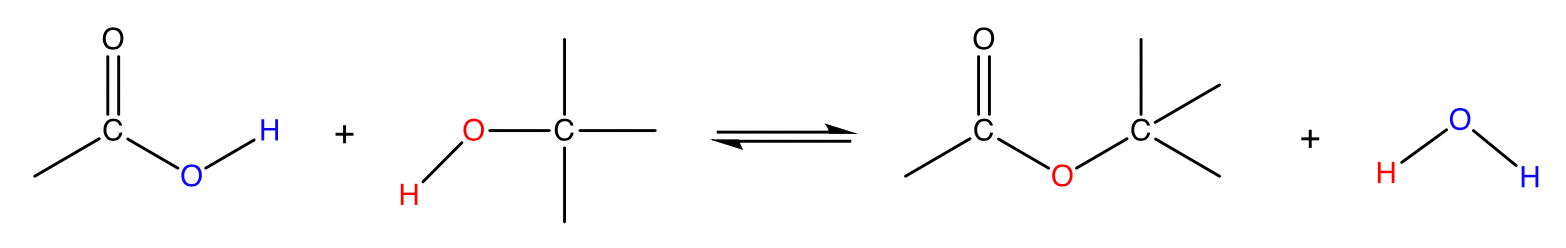
Conditions: Concentrated \small H_2SO_4 catalyst, heat
Other Organic Reactions
Fermentation of glucose
glucose → ethanol + carbon dioxide
\small C_6H_{12}O_{6(aq)}\ \ \underrightarrow{ \ zymase \ \ } \ \ 2C_2H_5OH_{(aq)}+2CO_{2(g)}
Fermentation involves following four conditions.
- Presence of an enzyme called zymase (found in yeast)
- Warm temperatures (30–40 °C)
- Anaerobic environment (absence of oxygen)
- Aqueous solution
Reaction pathway questions
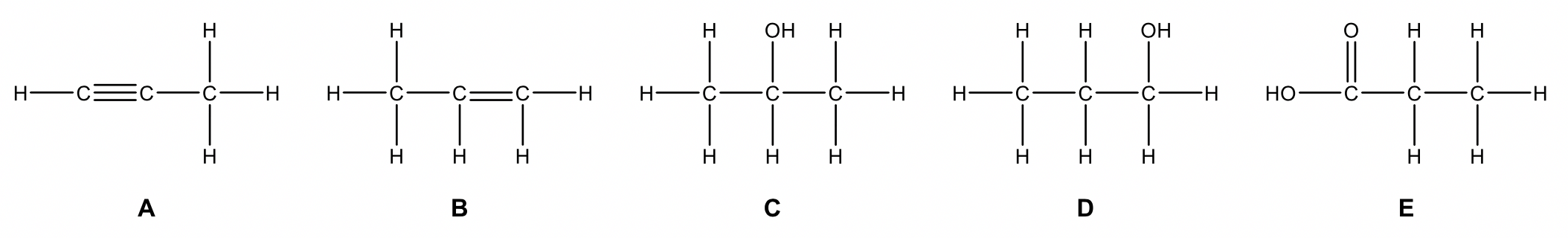
Question 1
The reaction pathway below represents the synthesis of compound E. Only one organic product is formed in each step (no isomeric products possible).
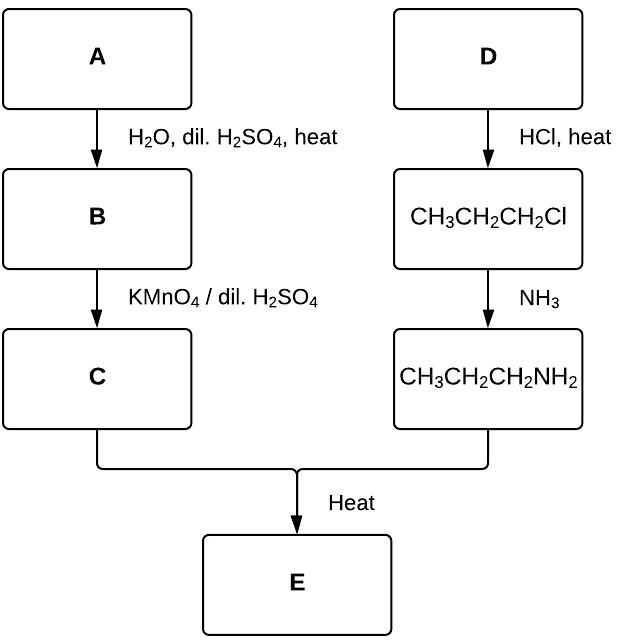
Draw the structures of compounds A to E, justifying your diagrams with reference to the conditions provided. (5 marks)
Question 2
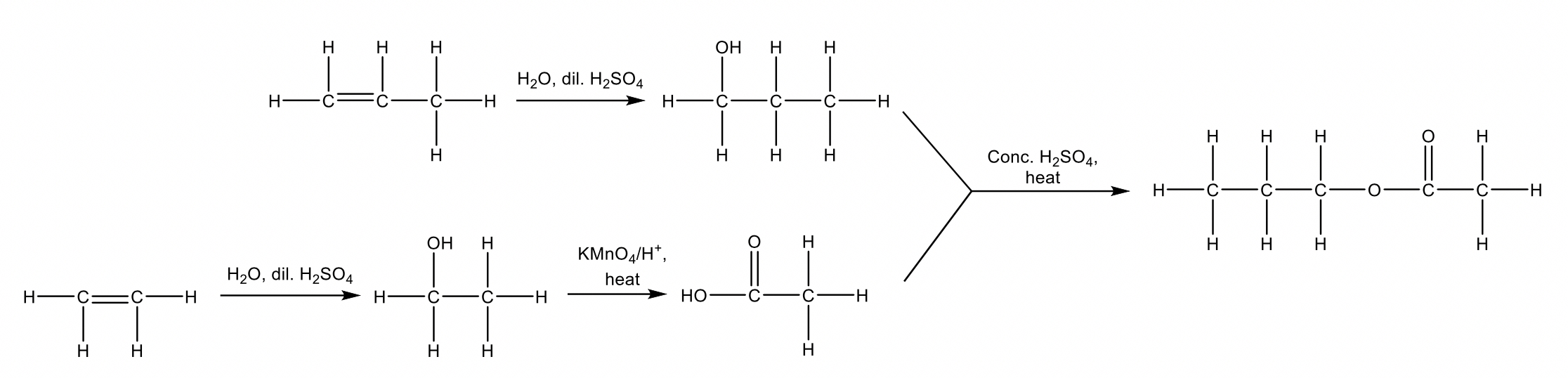
The flowchart represents the reaction pathways to synthesise compound E from compounds A and B.
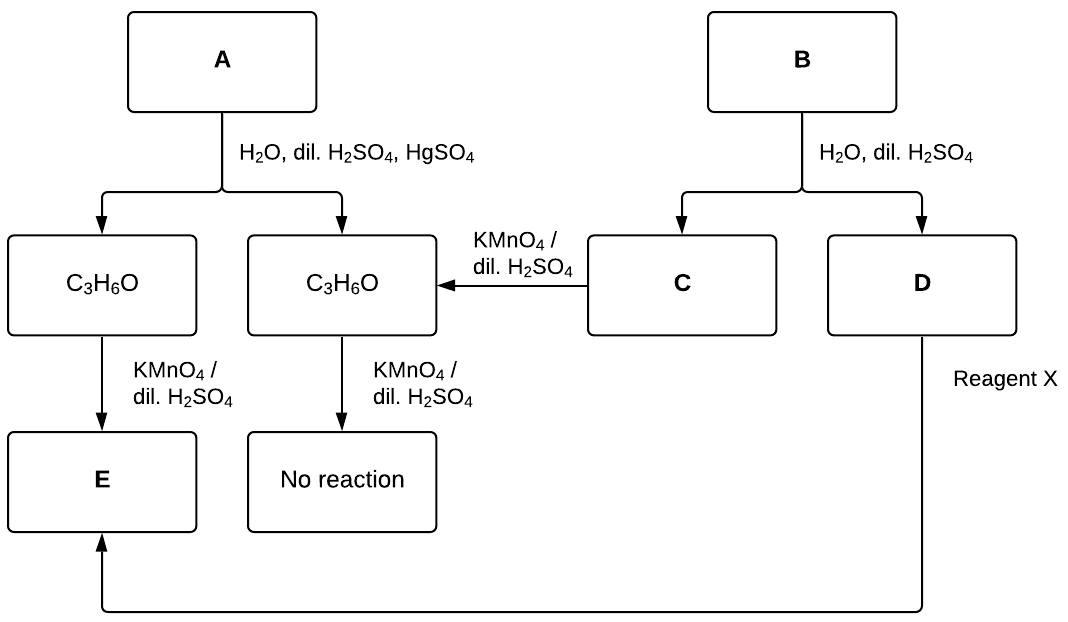
Identify reagent X and draw the structures of compounds A to E, justifying your diagrams with reference to the conditions provided. (6 marks)
Question 3
Devise reaction pathways for the synthesis of the following compounds from the given starting material. More than one step is required. Indicate the reagents required and draw the structural formulae of any intermediate compounds.
| a) | Propan-2-ol from propane | 3 marks |
| b) | Propyl ethanoate from propene and ethene | 5 marks |
Solutions to reaction pathway questions
| Q | Answer |
| 1 | 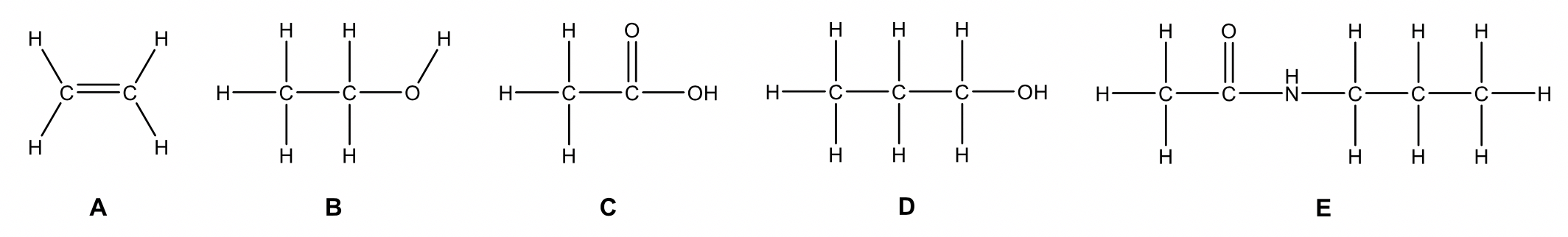 |
| 2 | Reagent X is acidified KMnO4
|
| 3 | a)
b)
|
Looking for more HSC Chemistry practice questions?
Now that you have mastered all the organic chemical reactions you need to know for the HSC, refine your exam skills by practicing these 20 Must Know HSC Chemistry Questions.
Learnable Education and www.learnable.education, 2019. Unauthorised use and/or duplications of this material without express and written permission from this site's author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Learnable Education and www.learnable.education with appropriate and specific direction to the original content.