In this article
Glossary of Chemistry Terms Used in the HSC
This complete glossary of Chemistry terms lists all the terminology you need to ace HSC Chemistry.
What is glossary of Chemistry terms?
This complete glossary of Chemistry terms provides a quick reference to the definitions of commonly used chemistry terms in HSC Chemistry course.
Go to Chemistry term:
A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
Acid dissociation constant
The acid dissociation constant, given the symbol Ka, is the equilibrium constant for the dissociation (ionisation) of an acid into a hydrogen ion and an anion.
HA(aq) \rightleftharpoons H+(aq) + A–(aq)
K_a = \dfrac{[H^+][A^-]}{[HA]}
Addition polymer
Addition polymers are polymers formed from the addition reaction of two unsaturated monomers, without the elimination of any atoms. Examples of addition polymers include high density polyethylene (HDPE), low density polyethylene (LDPE) and polyvinyl chloride (PVC).
Alcohols
Alcohols are organic compounds which contain a hydroxyl (O-H) group.
Aldehyde
Aldehydes are organic compounds which contain a carbonyl functional group on a terminal carbon of a carbon chain. A carbonyl functional group consists of a carbon atom attached to an oxygen atom by a double bond. C=O
Aliquot
An accurately known volume of a liquid. In titration this usually refers to the liquid transferred via pipette into the conical flask.
Alkanes
Alkanes are saturated hydrocarbons containing only single bonds.
They have the general formula:
C_nH_{2n+2}
Alkenes
Alkenes are unsaturated hydrocarbons which contain carbon-carbon double bonds.
They have the general formula:
C_nH_{2n}
Alkynes
Alkynes are saturated hydrocarbons which contain carbon-carbon double bonds.
They have the general formula:
C_nH_{2n-2}
Amides
Amides are carboxylic acids where the OH group has been replaced with an amine NH2. They use the suffix “-amine”
Amines
Amines are organic compounds which contain the amino (-NH2) group. They use the suffix “-amine”
Amphoteric substances
Amphoteric substances can react with both acids and bases. Examples of amphoteric substances are H2O, Al2O3
Amphiprotic substances
A molecule or ion that can accept and donate protons. Amphiprotic species will lose or gain a proton depending on the other reactant.
- If the other species is a stronger base, it will actas a Brønsted Lowry acid
- If the other species is a stronger acid, it will act as a Brønsted Lowry base
Amphiprotic substances are a class of amphoteric substances.
Analyte
The analyte refers to the chemical species that is to be measured or analysed. In titrations, the analyte refers to the solution of unknown concentration.
Atomic absorption
Atomic absorption occurs when electrons in the ground state absorb light energy corresponding to the energy difference between levels and are excited to a higher energy level.
Absorption spectra appear as black lines on a coloured background. The absorption spectrum of hydrogen is shown below.

Back Titration
A two-stage analysis in which an excess of reactant is added to the analyte, then the excess is determined to calculate the concentration of the analyte.
Beer-Lambert Law
Beer-Lambert law states that absorbance is proportional to both concentration and length of the sample.
A = \epsilon l c
\epsilon : Molar absorptivity (also known as extinction coefficient), a constant that relates absorbance, concentration and path length, with the units L cm-1 mol-1
l: Path length of the sample in cm
c: Concentration of the substance in the sample in mol L-1
Biofuels
Biofuels are fuels derived from biomass which are biological material from living or recently living organisms. The 3 main types of biofuels are:
- Bioethanol: Ethanol produced from the fermentation of monosaccharides such as glucose and fructose.
- Biogas: Consists of a mixture of gases, such as methane, carbon monoxide and hydrogen, released by the natural breakdown of organic matter by anaerobic bacteria.
- Biodiesel: A liquid fuel composed of a mixture of long hydrocarbon chain esters.
Buffers
A buffer solution resists changes in pH when small amounts of acids and bases are added.
They are commonly made by mixing a weak acid and its conjugate base. (or a weak base and its conjugate acid)
Buffer capacity refers to the effectiveness of a buffer and is defined as the moles of H3O+ or OH- necessary to change the pH of a buffer solution by one unit. Buffer capacity depends on both the pH of the buffer and the total concentration of the weak acid and conjugate base.
Burette
A graduated piece of glassware which dispenses measured amounts of solution.
![]()
Carboxylic acids
Carboxylic (alkanoic) acids contain the carboxyl functional group which consists of a carbonyl group, C=O, connected to a hydroxyl group, -OH. The carboxyl group is abbreviated as COOH and is given the suffix “-oic acid”
Chromophore
A chromophore is a structural fragment that absorbs a characteristic wavelength.
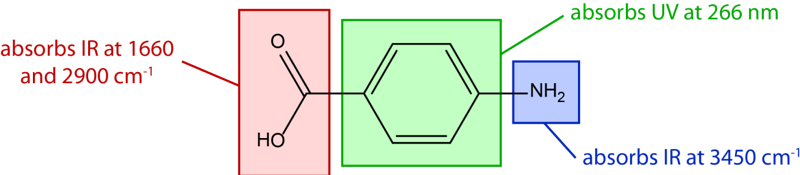
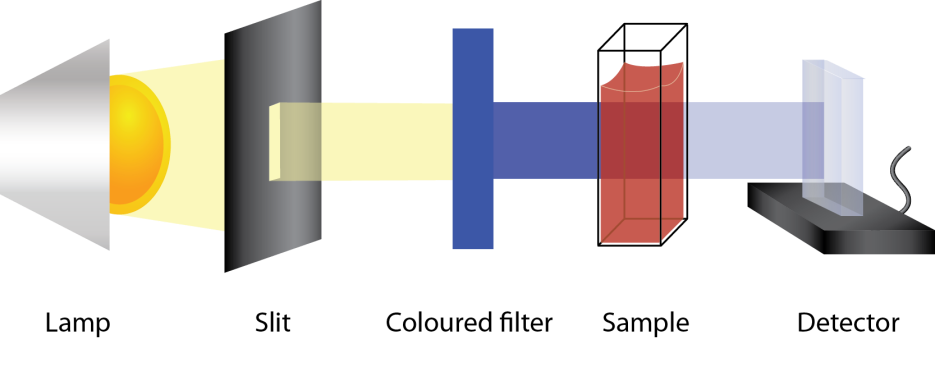
Colourimetry
Colourimetry is an analytical technique used to determine the concentration of a coloured substance in a solution based on its absorbance of light.
Colourimetry operates on the principle of Beer Lambert law: the absorbance of light is directly proportional to the concentration of analyte present in the sample ( A = \epsilon l c ). Therefore, by determining the absorbance of light using a colourimeter, we can determine the concentration of the coloured solution.
Below is a schematic for a colourimeter:
Condensation polymer
Condensation polymers are formed through the condensation reaction of two difunctional monomers with the elimination of a small molecule such as water or methanol in the process.
Nylon-6,6 is an example of a condensation polymer.
Conjugate acid/base pairs
When a Brønsted Lowry acid loses a proton, it forms the conjugate base of that acid.
When a Brønsted Lowry base gains a proton, it forms the conjugate acid of that base.
For example, in the following equilibrium
HA(aq) \rightleftharpoons H+(aq) + A–(aq)
Two species in a conjugate pair have inverse strength.
Conjugation
Molecules that have conjugation have alternating double (or triple) and single bonds. In ultraviolet-visible spectroscopy, the presence of strong absorption bands in the spectrum above 200nm can indicate the presence of conjugation in a molecule.
Coordination complex
A coordination complex consists of a central metal ion (typically a transition metal) coordinated to molecules or ions. These molecules or ions are called ligands and are typically neutral or negatively charged.
Density
The density of a substance is the measure of its mass per unit volume.
d = \dfrac{m}{v}
The unit for density is gmL-1 or gcm-1
Destructive testing
Destructive testing refers to any chemical or analytical test in which the sample being tested is destroyed in the process and therefore cannot be recovered.
Dipole-dipole forces
Dipole-dipole forces are electrostatic attractions between molecules with permanent dipoles (polar molecules): the positive end of one molecule attracts the negative end of another molecule, aligning the molecules.
Dispersion forces
Dispersion forces are the forces of attraction between fluctuating dipoles in atoms and molecules that are very close together.
Effervescence
Effervescence refers to the appearance of bubbles which indicates a reaction has occurred.
Electron shielding in NMR
Since electrons are moving charged particles, they create a small magnetic field around a nucleus. This changes the difference in energy between the two nuclear spin states and hence the absorbed frequencies during nuclear magnetic resonance spectroscopy. This is known as electron shielding.
Empirical formula
The empirical formula for a compound gives the simplest integer ratio of atoms of each element in a compound. For example the empirical formula of octane which has the molecular formula C8H18 is C4H9.
End point
The end point of a titration is when the indicator first undergoes a permanent colour change.
Enthalpy
Enthalpy (with the symbol H) is a measure of the heat content of a system. While absolute enthalpy cannot be measured, the change in enthalpy \Delta can be measured.
\Delta H is usually given in kilojoules per mole (kJ mol-1)
\Delta H = H_{products} - H_{reactants}
Entropy
Entropy (with the symbol S) is a measure of the dispersion of available energy. It is also sometimes referred to as a measure of disorder.
\Delta S = S_{products} - S_{reactants}
A greater dispersal of energy or greater disorder will increase entropy.
Equivalence point
The equivalence point (stoichiometric point) is when exactly enough moles of titrant have been added to react with all of the titrant, without any excess of either.
Ester
Esters are organic compounds with the functional group (-COO-)
They are formed from an esterification reaction between a carboxylic acid and an alcohol. During this reaction, the O-H group on the acid is replaced with an O-R group from the alcohol.
Fermentation
Fermentation is a process that involves the conversion of carbohydrates into simple alcohols by the action of enzymes (biological catalysts) produced by microorganisms such as yeast and bacteria.
Example of the fermentation of glucose to produce ethanol:
glucose → ethanol + carbon dioxide
C6H5O6 (aq) → 2C2H5OH (aq) + 2CO2 (g)
The four conditions that must be met for this fermentation:
- The presence of a the zymase (found in yeast)
- Warm temperatures (30 – 40 ºC)
- Anaerobic environment (without oxygen)
- Aqueous solution of sugar
Fingerprint region
The fingerprint region of an infrared spectrum refers to the region between 1500 – 666 cm-1.
Many overlapping signals are present in this region, leading to unique spectra for different molecules. Peaks in this region are not normally interpreted in detail but comparison of this region with database of standards allows for identification of specific compounds. This is because each molecule has a unique fingerprint region.
Functional group
A functional group is an atom, or groups of atoms which give a compound characteristic physical and chemical properties. They are sites of chemical reactivity.
Eg. carbon-carbon double/triple bonds, hydroxyl groups, carboxyl groups
Gravimetric analysis
Gravimetric analysis is a quantitative analytical technique which uses the mass of products formed through a precipitation reaction to quantify an analyte.
Gravimetric analysis is typically performed with the following steps
- A known quantity of sample is dissolved in water
- The analyte is precipitated from solution using a suitable reagent
- The precipitate is collected by filtration, then washed, dried and weighed.
Halogenated organic compound
Halogenated organic compounds refer to compounds formed when one or more hydrogen atoms on a hydrocarbon have been replaced with a halogen.
Homologous series
Homologous series refer to groups of molecules which have a similar structure and the same general formula. That is, each member of a homologous series differs from the previous member by a -CH2- unit. Molecules of the same homologous series also has similar chemical properties and a pattern to their physical properties.
Hydrogen bonding
Since fluorine, oxygen and nitrogen are very electronegative, and hydrogen is very electropositive, these atoms can combine to form an extremely strong intermolecular interaction known as hydrogen bonding. (H-bonding)
Hydrogen bonding occurs between:
- hydrogen atoms bonded to nitrogen, oxygen or fluorine atoms in one molecule, and;
- a non-bonding (lone) electron pair on the nitrogen, oxygen or fluorine atom in an adjacent molecule.
Hygroscopic
Hygroscopic substances absorb water from the air and can change weight during weighing. Hygroscopic substances are not suitable for use in the preparation of a primary standard. Examples of hygroscopic substances include NaOH and KOH.
Indicator
Indicators give a qualitative indication of the concentration of hydrogen ions in a solution. Most indicators are weak acids or bases. This means that there is an equilibrium between its two forms.
Isotopes
The isotopes of an element have the same atomic number (proton number) but a different number of neutrons and hence a different mass number (protons + neutrons).
Ketone
Ketones are organic compounds which contain a carbonyl functional group in the middle of a carbon chain. A carbonyl functional group consists of a carbon atom attached to an oxygen atom by a double bond. C=O
Markovnikov’s Rule
Markvonikov’s rule states that: “In addition reactions involving unsymmetrical alkenes, the hydrogen atom will predominantly bond to the carbon atom bearing the greater number of hydrogen atoms”
This rule can be used to predict the major and minor product formed when asymmetric reagents, such as HBr, reacts with an asymmetric alkene.
Molecular formula
Molecular formula gives the actual number of atoms of each element in a molecule or compound
Parallax error
Parallax error occurs when viewing an object at a different angle results in a different measurement. To avoid parallax error, take all measurements at eye level.
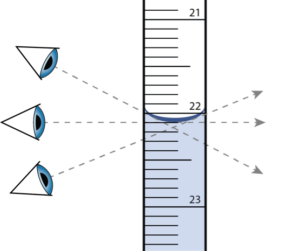
Pipette
A piece of glassware used to transfer a very accurately measured volume of solution. Pipettes uses in titration are calibrated to deliver the specified volume of solution with no additional force. Therefore, any liquid left on the tip of the pipette should not be shaken or blown out.
![]()
Polymer
A polymer is a long chain molecule made up of repeating units called monomers, joined by covalent bonds.
Polyprotic acids
Polyprotic acids are acids that can produce more than one H+ ion.
- Diprotic acids can produce 2 H+ ions
- Triprotic acids can produce 3 H+ ions
Purity
Purity refers to the amount of desired product in a sample expressed as a percentage. It is commonly used as a measure of the quality of a product.
Percentage purity (%) = \dfrac{Mass of desired product (g)}{Total mass of sample (g)} × 100
Redox Reactions
Redox reactions (oxidation and reduction) involve the transfer of electrons.
The chemical species being oxidised loses electrons and the one being reduced gains electrons.
Reflux
Reflux is a technique that involves heating a reaction mixture in a vessel fitted with a cooling condenser so that the volatile reactants and products are returned to the reaction mixture without any loss.
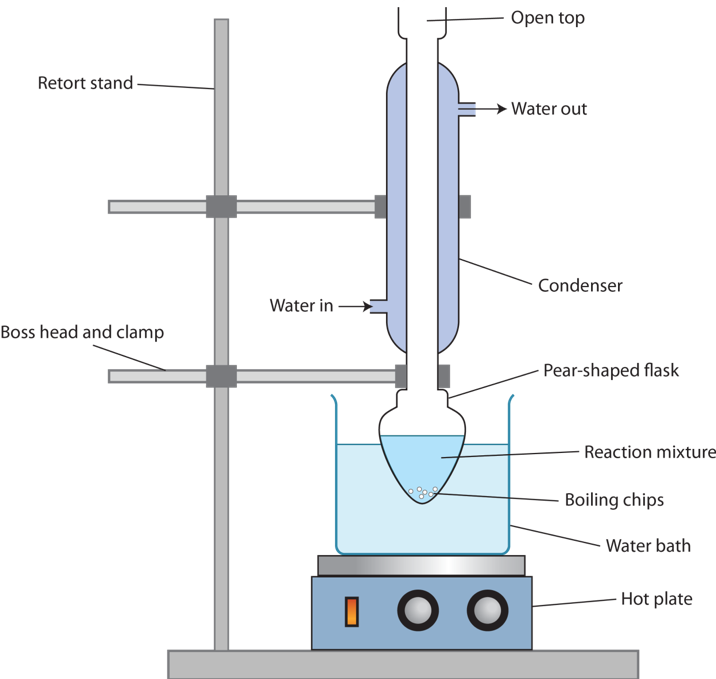
Reversible reaction
A reversible reaction is a reaction capable of proceeding in both the forward and reverse directions
Solute
A substance, usually a solid, which dissolves in a solvent to form a solution.
Solubility
A substance’s solubility is the maximum amount of solute that dissolves in a given amount of solvent, at a given temperature.
Surfactant
Surfactants or SURFace ACTive AgENTs function by reducing the surface tension of water and binding to grease and dirt to solubilise them.
Standard solutions
Standard solutions are solutions of accurately known concentration and composition often used in titrations. Standard solutions can be categorised as primary or secondary standards.
- Primary standards are produced when a substance of known high purity is dissolved in a known volume of solvent.
- Secondary standards are produced when its concentration is determined via stoichiometry.
Structural Isomers
Structural isomers are molecules that have the same molecular formula but are arranged in different ways, giving rise to different structural formulae.
Although they have the same molecular formula, they are different compounds with different chemical and physical properties as well as different names.
In HSC chemistry, we only look at three types of structural isomers:
- Chain isomers: Involve rearrangement of carbon atoms in the backbone resulting in a different number of carbons in the longest chain or different branching in the carbon chain
- Position isomers: result when molecules have the same carbon chain but the functional group is in a different location.
- Functional group isomers: result when atoms in the molecules are arranged in different ways that lead to the isomers having different groups.
Substitution reactions
Substitution reactions occur when an atom or functional group in a molecule is replaced or ‘substituted’ by another atom or group.
Titrand
The solution to which another reagent (the titrant) is added during a titration (usually in a conical flask)
Titrant
The solution that is added during a titration (usually from a burette).
Titration
Titration is an analytical process used to determine the moles of a substance (an analyte) present in a sample. The equipment used for a titration is shown below.
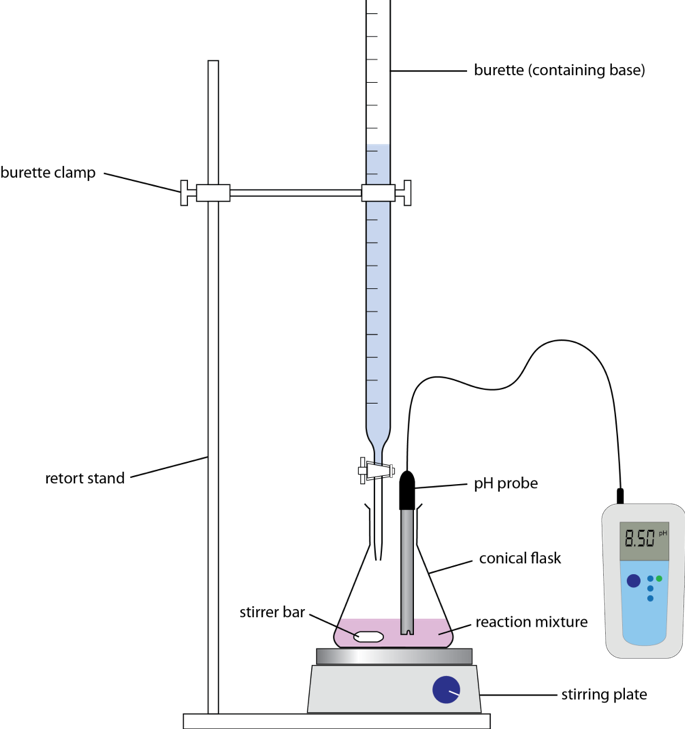
Titre
The volume of titrant used in a titration
Trace nutrients
Trace nutrients are nutrients we require in very small amounts (in the range of ppm). The levels of trace nutrients are important in many areas such as in soils, food, plants, blood and tissue. Inadequate or excessive amounts of trace nutrients can be harmful.
Viscosity
Viscosity refers to a substance’s resistance to fluid flow. Liquids with a relatively high resistance to flow have a high viscosity. Honey for example has a relatively high viscosity.
Volatility
Volatility is the ability of a liquid (or solid) to escape and form a vapour.
Volumetric flask
A volumetric flask is piece of glassware which can hold a set volume of solution very accurately. It is often used for the preparation of a standard solution.

Yield
The yield of a reaction is the amount of product obtained compared with the theoretical maximum.
% yield = \frac{actual}{theoretical} x 100
Looking for more tools to ace HSC Chemistry?
Check out our Chemical Reactions Cheatsheet and list of 20 Must Know HSC Chemistry Questions
Learnable Education and www.learnable.education, 2019. Unauthorised use and/or duplications of this material without express and written permission from this site's author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Learnable Education and www.learnable.education with appropriate and specific direction to the original content.