Ultimate Toolkit for HSC Chemistry Module 8 [Cheatsheet]
What do you need to know to ace HSC Chemistry Module 8 Applying Chemical Ideas?
As the module name suggests, a majority of HSC Chemistry Module 8 exam questions are application questions. This means that students must draw from their knowledge of various key chemical ideas to solve a problem.
In this article, you will find all the useful tables and chemical equations you need to analyse both inorganic and organic substances along with practice exam-style questions.
We discuss:
- Key chemical reactions
- Solubility rules
- Compound colours
- Characteristic flame test colours
- Basic and neutral ions
- Chemical tests for organic compounds
- Common mass fragments
- Infrared absorption data
- 13C NMR chemical shift data
- UV absorption
Key chemical reactions for HSC Chemistry Module 8
Acid-carbonate reaction
Acids react with metal carbonates to form water, carbon dioxide and a salt.
acid + carbonate → water + salt + carbon dioxide
eg. 2HCl(aq) + Na2CO3(aq) → H2O(l) + 2NaCl(aq) + CO2(g)
Since carbon dioxide is produced during this reaction, gas bubbles will be observed. This appearance of bubbles is referred to as effervescence and allows for carbonate anions (CO32-) to be easily identified through the addition of an acid.
Complexation reactions
Complexation reactions are often used to identify cations. The two complexation reactions you should memorise are:
- The reaction of iron (III) ions with thiocyanate ions to form a dark red complex.
- The reaction copper(II) ions with ammonia (NH3) to form a deep blue [Cu(NH3)4]2+ complex.
Solubility rules
Memorising the solubility rules is crucial to your success in HSC Chemistry.

Nitrate , ammonium and Group 1 salts (except ) are always soluble. You can recall most exceptions using the Ksp data in HSC Data Sheet.
Typically, the solubility of compounds is defined as follows:
- A soluble substance will dissolve more than 10 g/L.
- A slightly soluble substance will dissolve at 1-10 g/L.
- An insoluble substance will dissolve less than 1 g/L.
You are not expected to know the solubility of Hg2+ or Sr2+ salts for the HSC
Knowing the solubility rules will not only help you answer questions in HSC Chemistry Module 8, but will help you assign states when writing out chemical reactions.
Failing to memorise the solubility rules is one of the biggest mistakes students make going into their HSC Chemistry exam.
Compound colours
The colour of aqueous solutions and precipitates can indicate the presence of certain ions in an unknown sample.
The table below shows the colours of some common ionic compounds.
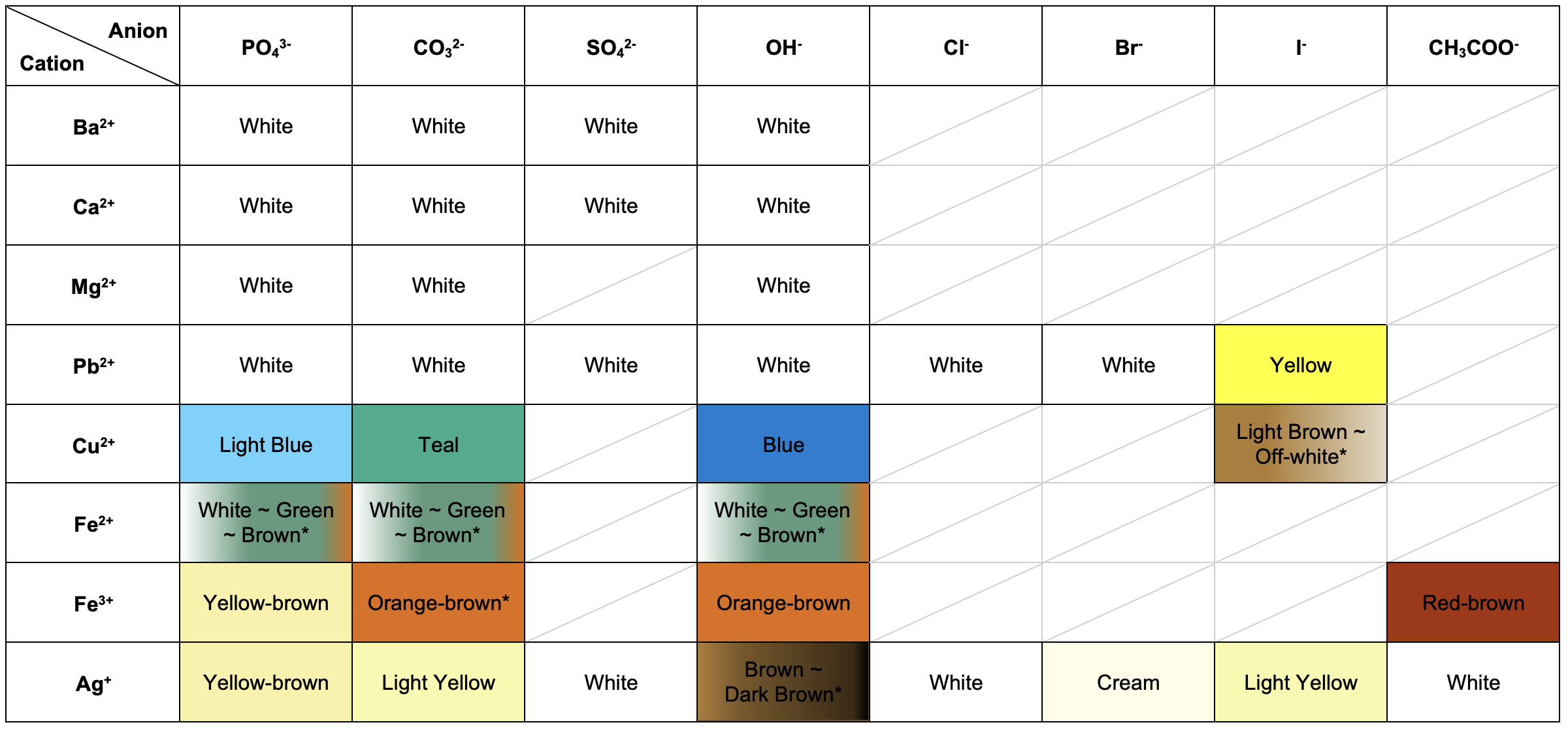
The colours of some precipitates are due to additional reactions (indicated by *), and these exceptions are summarised below.
- Copper(II) iodide: unstable, forming copper(I) iodide (off-white).
- Iron(II) phosphate/carbonate/hydroxide: the pure form is white, but in solution, they form basic iron(II)-iron(III) (green) and iron(III) hydroxide (orange-brown).
- Iron(III) carbonate: unstable, forming iron(III) hydroxide (orange-brown).
- Silver(I) hydroxide: unstable, decomposing to form silver(I) oxide (dark brown).
Ag+ halide precipitates decompose with exposure to light to form Ag(s). This is observed as darkening of the solid.
Question 1 (6 marks)
Write the net ionic equations for any precipitation reactions that may occur between the following compounds. If a precipitate is formed, identify its colour.
a) Ammonium nitrate + sodium phosphate
b) Lithium carbonate + iron(II) chloride
c) Potassium hydroxide + iron(III) chloride
Characteristic flame test colours
The following table outlines the flame test colours you are expected to know for HSC Chemistry Module 8.
| Metal Ion | Colour |
| Ba2+ | Pale green (apple green) |
| Ca2+ | Brick red (orange-red) |
| Pb2+ | Blue/grey-white |
| Cu2+ | Green (copper(I) or halide = blue-green) |
| Fe2+/Fe3+ | Orange-brown |
| Mg2+ | No characteristic flame colour* |
| Ag+ | No characteristic flame colour |
*Magnesium metal will give intense white colour as it reacts with oxygen.
Question 2 (6 marks)
A student performed some diagnostic tests to identify the cations of three nitrate solutions. Her observations are described below:
Solution Observations X Precipitate formed with sodium sulfate solution. An apple green colour resulted when solution X was sprayed into the Bunsen flame. Y Yellow precipitate formed with potassium iodide solution. The precipitate darkens with exposure to light. Z Precipitate formed with sodium hydroxide solution. An emerald green colour was observed when solution Z was sprayed into the Bunsen flame.
The possible cations are Cu2+, Na+, Pb2+, Ba2+, Ca2+ and Ag+. Identify the cation in solution X, Y and Z. Explain your answer and include relevant equations.
Basic and neutral ions
For anion identification, testing the pH of a solution can be used to narrow down the possible anions in a solution.
- Anions which are the conjugate bases of weak acids will produce a basic solution when dissolved in water.
- Anions which are the conjugate bases of strong acids will dissolve to produce a solution with a neutral pH.
This is summarised in the table below.
| Basic Ions | Neutral Ions |
| Hydroxide (OH–) Acetate (CH3COO–) Carbonate (CO32-) Phosphate (PO43-) | Chloride (Cl–) Bromide (Br–) Iodide (I–) Sulfate (SO42-) |
Question 3 (4 marks)
Identify a test that can be used to distinguish between the following pairs of ions and give the expected results.
a) Cl– and OH–
b) CO32- and PO43-
Chemical tests for organic compounds
To determine the presence of different functional groups, various chemical tests can be performed. These tests include the
- bromine water test,
- sodium metal test,
- reaction with acidified permanganate,
- litmus test and
- reaction with sodium carbonate.
The following table summarises the expected results of each test.
| Test | C=C double bond | Alcohol | Carboxylic acid |
| Bromine water | Decolourises | No immediate colour change (may change with heating but this can be ignored) | No colour change |
| Sodium metal test | No reaction | Effervesces | Effervesces |
| Reaction with acidified MnO4– | Decolourises | Decolourises | No colour change |
| Blue litmus | Stays blue | Stays blue | Turns red |
| Reaction with sodium carbonate | No reaction | No reaction | Effervesces |
The following flow chart illustrates a possible analysis sequence to distinguish between a carboxylic acid, alcohol, alkene and alkane using the tests outlined above.
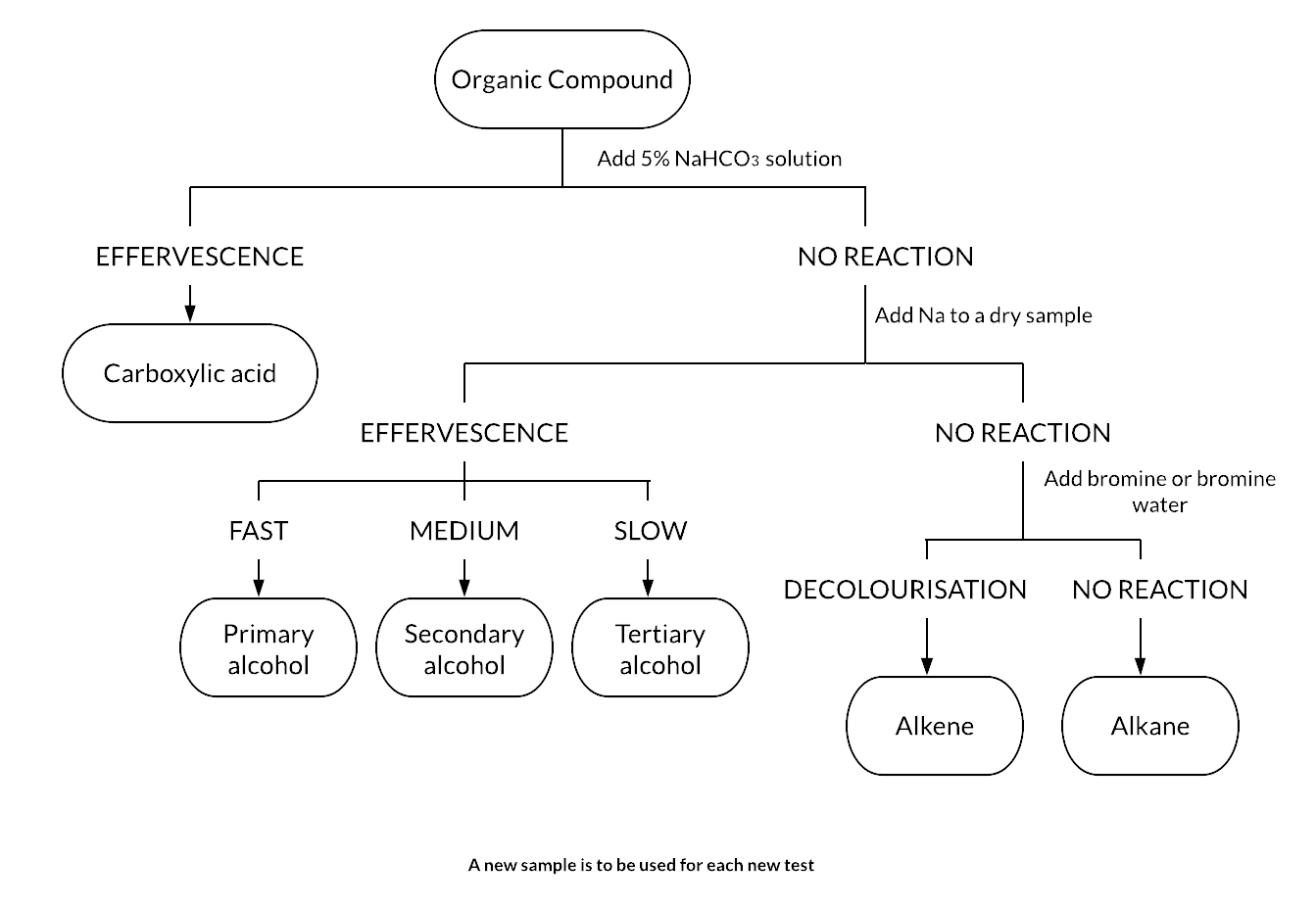
Question 4 (3 marks)
Describe a sequence of tests that could be performed to distinguish between pure samples of hex-2-ene, acetic acid and propan-1-ol.
Common mass fragments
The table below shows some commonly observed fragments in mass spectra.
| Mass | Fragment |
| 15 | 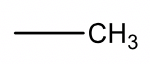 |
| 17 | 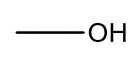 |
| 29 | 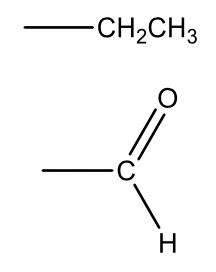 |
| 31 | 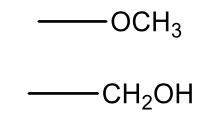 |
| 43 | 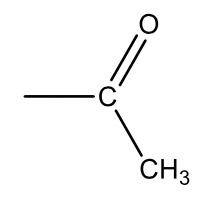 |
| 45 | 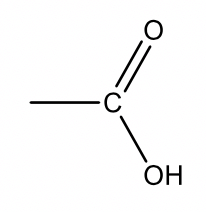 |
Infrared absorption data
| Bond | Wavenumber / cm-1 |
| N—H (amines) | 3300 – 3500 |
| O—H (alcohols) | 3230 – 3550 (broad) |
| C—H | 2850 – 3300 |
| O—H (acids) | 2500 – 3000 (very broad) |
| C\equivN | 2220 – 2260 |
| C=O | 1680 – 1750 |
| C=C | 1620 – 1680 |
| C—O | 1000 – 1300 |
| C—C | 750 – 1100 |
Question 5 (3 marks)
A sample of solvent was analysed by mass spectrometry and infrared spectroscopy. The following data were collected:
- Mass spectrum: peaks at m/z = 15, 17, 29, 46
- Infrared spectrum: broad peak at 3316 cm-1
Explain how the spectral data is evidence that the solvent is ethanol.
13C NMR chemical data shift
| Type of carbon | \delta/ppm |
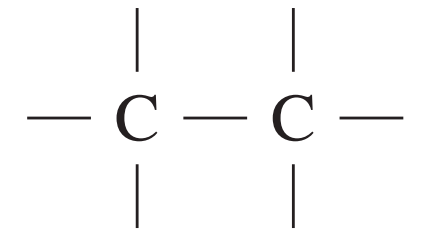 | 5 – 40 |
| 10 – 70 | |
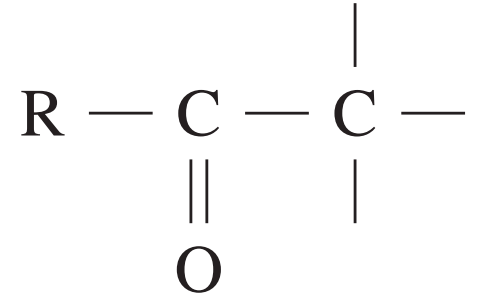 | 20 – 50 |
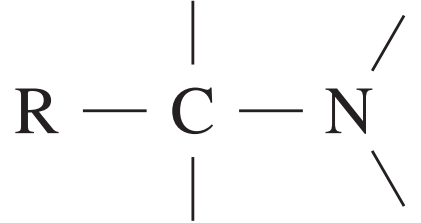 | 25 – 60 |
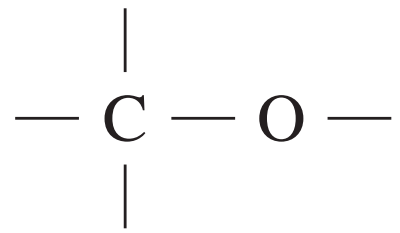 alcohols, ethers or esters | 50 – 90 |
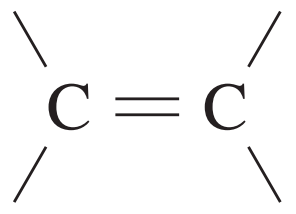 | 90 – 150 |
| 110 – 125 | |
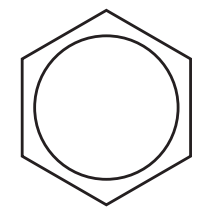 | 110 – 160 |
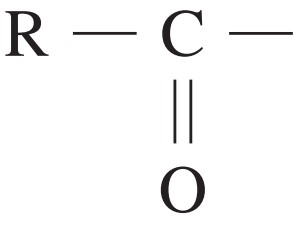 esters or acids | 160 – 185 |
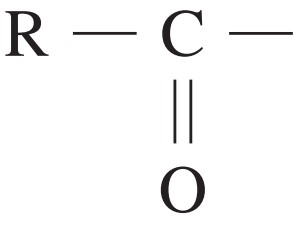 aldehydes or ketones | 190 – 220 |
UV absorption
(This is not a definitive list and is approximate.)
| Chromophore | \lambda_{max} (nm) |
| C—H | 122 |
| C—C | 135 |
| C=C | 162 |
| C\equivC | 173 178 196 222 |
| C—Cl | 173 |
| C—Br | 208 |
The last three tables can be found on the NESA HSC Chemistry Formula and Data Sheet or on Learnable’s online, mobile friendly version.
Solutions to HSC Chemistry Module 8 practice questions
Access over 1000+ HSC Chemistry Exam-Style Questions on Learnable.
Test your understanding of any HSC Chemistry concepts with Learnable’s customisable quizzes. Try Learnable for free now.
- Instant feedback on syllabus specific questions
- Step-by-step detailed solutions
- Intelligent reporting
Learnable Education and www.learnable.education, 2019. Unauthorised use and/or duplications of this material without express and written permission from this site's author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Learnable Education and www.learnable.education with appropriate and specific direction to the original content.