In this article
Year 11 Chemistry Practical Investigation | Calorimetry Experiment
Have a chemistry practical on the calorimetry experiment? Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment.
How to perform the calorimetry experiment in Year 11 Chemistry Practical
A popular Year 11 Chemistry practical investigation is the calorimetry experiment. Not only is this experiment commonly performed by students during their Year 11 Chemistry course but also in the HSC Chemistry course. In this article, you will find a complete Chemistry practical report on determining the enthalpy of combustion of fuels via calorimetry.
This Year 11 Chemistry practical report on the calorimetry experiment consists of:
- Aim
- Theory
- Materials
- Safety information
- Method
- Results
- Calculations
- Quantitative analysis
- Qualitative analysis
- Practice calorimetry problems
Calorimetry Experiment
Aim
To determine the enthalpy of combustion of fuels using a calorimeter.
Theory
The standard enthalpy of combustion \small (\Delta H_c^\circ) is the enthalpy change when one mole of a substance undergoes complete combustion with oxygen at standard states, under standard conditions.
The steps which can be used to determine the enthalpy changes of combustion are outlined below:
Step 1: Write a balanced chemical equation of the process.
\large \text{Fuel}_{(s)/(l)/(g)} + {O_2}_{(g)} \rarr {CO_2}_{(g)} + {H_2O}_{(l)}
Step 2: Calculate the heat gained by the substance (water).
\Large q_\text{ substance} =mc\Delta T
where:
- \small q_\text{ substance} is the heat gained by water in joules \small \text{(J)}
- \small \text{m} is the mass of water in kilograms \small (\text{kg})
- \small \text{c} is the specific heat capacity of water (4.18 × 103 \small \text{J kg}^{-1}\text{K}^{-1} or 4.18 \small \text{J g}^{-1}\text{K}^{-1} )
- \small \Delta \text{ T} is the change in temperature of water in kelvin \small \text{(K)}
Step 3: Calculate the heat released by the combustion process.
The quantity of heat exchanged between the process and the substance will be the same but opposite sign.
\Large q_\text{ combustion process} = -q_\text{ substance}
Step 4: Calculate the enthalpy change of the process.
To calculate the standard enthalpy of combustion from the results of a calorimetry experiment:
\Large \Delta H_c = \dfrac{q_\text{ combustion process}}{n_\text{ combustion process}}
Since the standard enthalpy of combustion of fuels (∆Hc) is always negative, we often use the term “heat of combustion”. The heat of combustion is the absolute value of the standard enthalpy of combustion, as the amount of heat released for a specified amount of the fuel.
The equation q=mc\Delta T and the value for the specific heat capacity of water can be found on the HSC Chemistry Formula and Data Sheet.
The fuels used during this experiment are alcohols which have a general formula:
\Large C_nH_{2n+1}OH .
For example,
- Methanol has the formula \small CH_3OH .
- Ethanol has the formula \small C_2H_5OH .
- Propanol has the formula \small C_3H_7OH .
Materials
- Copper can
- Retort stand and clamp
- Glass rod
- Methanol spirit burner
- Ethanol spirit burner
- Propan-1-ol spirit burner
- 250 \small \text{mL} measuring cylinder
- Thermometer (0 – 100 \small \degree \text{C} accurate to 0.1 \small \degree \text{C} )
- Electronic balance
Safety Information
| Material Used | Hazard | Precautions |
| Methanol | Toxic by all routes of exposure, if ingested causes permanent blindness, highly flammable | Wear eye and skin protection |
| Ethanol | Highly flammable, slightly toxic if ingested | Wear eye protection |
| Propan-1-ol | Highly flammable, toxic if ingested or inhaled | Wear eye protection |
Method
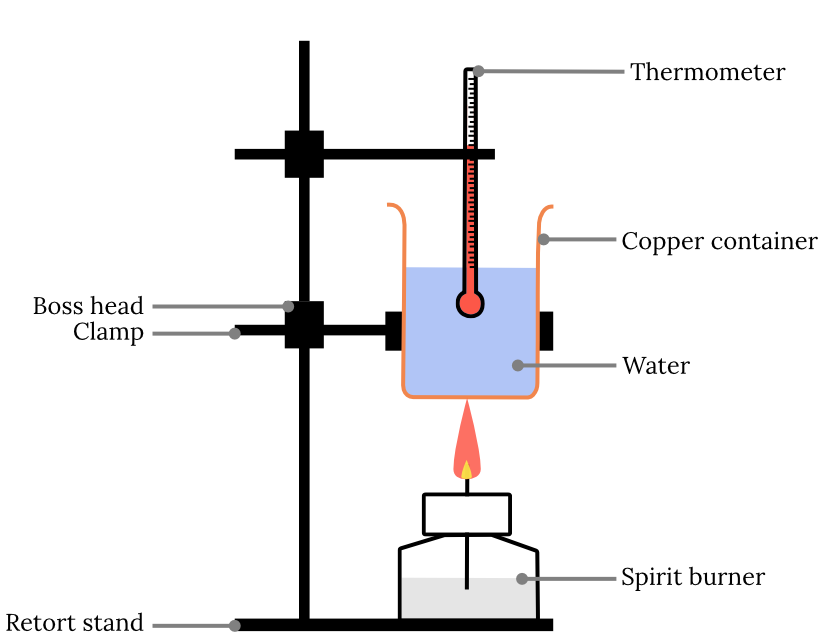
- Set up the equipment as shown below. The copper can should be clamped so that the tip of the flame just touches the can when lit.
- Add 200 \small \text{mL} of cold water to the can using a measuring cylinder to measure the volume of water.
- Record the initial temperature of the water using a thermometer.
- Weigh the spirit burner with its liquid contents as accurately as possible, and record the mass.
- Light the wick and stir the water gently with a glass rod. Monitor the temperature and observe the flame.
- When the temperature has risen by about 10 \small \degree \text{C} , extinguish the flame by replacing the cap.
- Accurately record the maximum temperature for the water.
- Reweigh the burner and record its final mass.
- Examine the bottom of the can for soot accumulation. Remove soot before using the next alcohol.
Results
Table 1: Experimental measurements and observations
| Measurement | Methanol | Ethanol | Propan-1-ol |
| Initial temperature of water ( \small \degree \text{C} ) | 25.0 | 25.0 | 25.0 |
| Final temperature of water ( \small \degree \text{C} ) | 35.0 | 35.0 | 35.0 |
| Initial mass of spirit burner ( \small \text{g} ) | 110.0 | 110.0 | 110.0 |
| Final mass of spirit burner ( \small \text{g} ) | 109.45 | 109.55 | 109.57 |
| Mass of fuel burned ( \small \text{g} ) | 0.55 | 0.45 | 0.43 |
| Observation of flame colour | Blue | Blue-yellow | Orange |
| Observation of soot deposition | No | Yes | Yes |
Calorimetry formulas
\Large q_\text{ substance} =mc\Delta T
\Large q_\text{ combustion process} = -q_\text{ substance}
\Large \Delta H_c = \dfrac{q_\text{ combustion process}}{n_\text{ combustion process}}
Calorimetry calculations
An example of the calculation of the enthalpy of combustion of methanol \small (CH_3OH) is shown below.
Step 1: Write a balanced chemical equation of the process.
{CH_3OH}_{(l)} + \frac{3}{2} {O_2}_{(g)} \rarr 2{H_2O}_{(l)} + {CO_2}_{(g)}
Step 2: Calculate the heat gained by the water.
\begin{aligned} q_\text{ substance} & = mc\Delta T \\ \\ & = 200 \text{ kg} \times (4.18 \times 10^{-3} \text{J kg}^{-1}\text{K}^{-1}) \times (35.0-25.0) \text{ K} \\ \\ &= 8360 \text{ J} \end{aligned}
Step 3: Calculate the heat released by the combustion process.
\begin{aligned} q_\text{ combustion process} &= -q_\text{ substance} \\ \\ &=-8360 \text{ J} \\ \\ &=-8.360 \text{ kJ} \end{aligned}
Step 4: Calculate the enthalpy change of the process.
\begin{aligned} n(CH_3OH) & = \dfrac{n}{MM} \\ \\ &=\dfrac{0.55}{12.01+4\times 1.008+16} \\ \\ &=0.01716 \dots \text{ mol} \end{aligned}
\begin{aligned} \Delta H_c&=\dfrac{q_\text{ combustion process}}{n_\text{ combustion process}} \\ \\ & = -\dfrac{8.36}{0.01716 \dots} \\ \\ & = - 490 \text{ kJ mol}^{-1} \text{(2 s.f.)} \end{aligned}
A summary of the calculations for the enthalpy of combustion of methanol, ethanol and propan-1-ol is displayed in the table below.
Table 2: Calculations
| Calculated Quantity | Methanol | Ethanol | Propan-1-ol |
| \small \Delta T : Change in temperature \small (\degree \text{C}) | 10.0 | 10.0 | 10.0 |
| \small m : mass of water heated \small (\text{g}) | 200 | 200 | 200 |
| \small q_\text{ substance} : quantity of heat gained by water \small (\text{kJ}) | 8.36 | 8.36 | 8.36 |
| \small q_\text{ combustion process} : quantity of heat released by the combustion process \small (\text{kJ}) | -8.36 | -8.36 | -8.36 |
| Mass of fuel burned \small (\text{g}) | 0.55 | 0.45 | 0.43 |
| \small MM : Molar mass of fuel \small (\text{g mol}^{-1}) | 32 | 46 | 60 |
| \small n : moles of fuel burnt \small (\text{mol}) | 0.017 | 0.0098 | 0.0072 |
| Enthalpy of combustion \small (\text{kJ mol}^{-1}) | – 490 | – 860 | – 1200 |
Discussion
Students are often asked to answer the following quantitative and qualitative analysis questions after performing a chemistry practical on calorimetry.
Quantitative analysis
1. The theoretical value for the enthalpy of combustion of each alcohol is given in the table below.
| Fuel | Experimental value for \small \boldsymbol{\Delta H_c \ (\textbf{kJ mol}^{-1})} | Theoretical value for \small \boldsymbol{\Delta H_c \ (\textbf{kJ mol}^{-1})} |
| Methanol | – 490 | – 726 |
| Ethanol | – 850 | – 1368 |
| Propan-1-ol | – 1200 | – 2021 |
Calculate the percentage error of the experimental result compared to the theoretical result for each alcohol.
\% \text{ error} = \dfrac{| \text{theoretical value} - \text{experimental value}|}{\text{theoretical value}} \times 100
\begin{aligned} \% \text{ error}_\text{ methanol} &= \dfrac{|-726 -(-490)|}{726} \times 100 \\ \\ & = 32.5 \% \\ \\ \% \text{ error}_\text{ ethanol} &= \dfrac{|-1368 -(-850)|}{1368} \times 100 \\ \\ &=37.9\%\\ \\ \% \text{ error}_\text{ propan-1-ol} &= \dfrac{|-2021 -(-1200)|}{2021} \times 100 \\ \\ &=40.6 \% \end{aligned}
Qualitative analysis
Let’s investigate the safety, errors, reliability and accuracy of this experiment.
1. Outline two safety risks in this experiment. Describe how the risks were minimised.
| Source of risk | Potential hazard | Procedure to minimise risk |
| Highly flammable alcohols | Can ignite and cause unwanted fires. |
|
| Heating equipment | Touching hot equipment can cause burns. |
|
2. Account for the differences between the experimental value and the theoretical values for the standard enthalpies of combustion.
In the experiment, not all of the heat produced was used in heating the water. Some of the heat was lost to the surroundings (heating the copper can and the air around the flame). Since this was not taken into account in the calculations, it caused the experimental value for the enthalpy of combustion to be significantly higher than the theoretical value.
3. Assess the validity of this experiment
Validity relates to the experimental method and how appropriate it is in addressing the aim of the experiment.
The aim of this experiment was to determine the enthalpy of combustion of fuels using a calorimeter. Therefore, the validity of the experiment can be assessed based on how suitable the method was in determining the enthalpy of combustion of each fuel.
- The enthalpy of combustion of a fuel refers to the energy released in the complete combustion of one mole of fuel. Therefore the fuels should have undergone complete combustion. However, this is not true for ethanol and propan-1-ol as black soot was observed on the base of the copper can. Black soot which is C(s) is an indication of incomplete combustion. The blue-yellow and orange flame observed during the combustion of ethanol and propan-1-ol is also an indication of incomplete combustion.
- The calculation of enthalpy made in this experiment assumes that there is no heat loss. However, this assumption is not satisfied as considerable heat is lost to the surroundings.
Since the experimental method contains assumptions that are not valid, the experiment is not valid.
4. Suggest techniques that could be used to improve the validity of the results.
The validity of an experiment can be improved by:
- Keeping the control variables constant and preventing them from affecting the dependent variable.
- Ensuring that any assumptions made are valid.
| Assumption | How to ensure the assumption is satisfied |
| No heat loss to the surroundings |
|
| The fuels undergo complete combustion |
|
5. What can be done to ensure the reliability of the results?
Reliability is the extent to which the experiment yields the same result each time.
The reliability can be improved by conducting more trials for each fuel, excluding outliers and averaging concordant results. Using a greater volume of water and recording results over a larger change in temperature will also reduce the percentage error, minimise the effect of random errors and improve the reliability of the results.
6. What can be done to improve the accuracy of the results?
Accuracy is the extent to which the calculated value differs from the true, accepted value.
Eliminating systematic errors arising from the incorrect use of equipment or improper calibration of instruments such as zero setting error (where the instrument does not read zero when the quantity to be measured is zero) will improve accuracy.
| Type of error | How to mitigate this error and improve accuracy |
| Parallax error when measuring volume | Take the reading with the line at eye level |
| Zero setting error in electronic balance | Tare the electronic balance before placing the spirit burner on it. |
Using more precise measuring devices will also improve accuracy. For example, use a 0–50 \small \degree \text{C} thermometer. It will be more accurate than a 0–100 \small \degree \text{C} one as the scale divisions are smaller. Alternatively, use a digital thermometer.
For more information on how to perform quantitative and qualitative data analysis on chemistry practical investigations, read the guide on How to study on data analysis task
Calorimetry problems
Question 1
A calorimetry experiment was conducted to determine the molar enthalpy of combustion of ethanol \small (C_2H_5OH) , molar mass = 46.07 \small \text{g mol}^{-1} ). The following data were collected:
| Initial mass of spirit burner | 250.35 \small \text{g} |
| Final mass of spirit burner | 249.84 \small \text{g} |
| Initial temperature of water | 20.4 \small \degree \text{C} |
| Final temperature of water | 35.8 \small \degree \text{C} |
| Mass of water | 152.1 \small \text{g} |
| a) | Calculate the heat absorbed by the water, and hence calculate the molar enthalpy of combustion of ethanol. | 3 |
| b) | It was estimated that 35% of the heat produced in the combustion reaction was lost to the surroundings in this experiment. What is the actual molar enthalpy of combustion for ethanol? | 3 |
Question 2
One method for improving the experimental design is to take into account the energy absorbed by the calorimeter. This energy is then added to the energy absorbed by the water to calculate the enthalpy of combustion.
Use the measurements provided below to calculate the approximate enthalpy of combustion of butan-1-ol (\small MM =74.12 \small \text{g mol}^{-1} ). (4 marks)
| Initial temperature of calorimeter | 20 \small \degree \text{C} |
| Final temperature of calorimeter | 50 \small \degree \text{C} |
| Volume of water | 250 \small \text{mL} (mass 1 \small \text{mL} = 1 \small \text{g} ) |
| Mass of calorimeter (without water) | 55.3 \small \text{g} |
| Specific heat capacity of calorimeter | 0.40 × 103 \small \text{J kg}^{-1}\text{K}^{-1} |
| Initial mass of spirit burner | 55.8 \small \text{g} |
| Final mass of spirit burner | 51.3 \small \text{g} |
Solutions to calorimetry problems
| 1 | Part a) Step 1: Write a balanced chemical equation of the process. {C_2H_5OH}_{(l)} + 3{O_2}_{(g)} \rarr 2{CO_2}_{(g)} + 3{H_2O}_{(l)} Step 2: Calculate the heat gained by the water. \begin{aligned} q_\text{ substance} & = mc\Delta T \\ \\ & = 0.1521 \text{ kg} \times (4.18 \times 10^{-3} \text{J kg}^{-1}\text{K}^{-1}) \times (35.8-20.4) \text{ K} \\ \\ &= 9790.98 \text{ J} \end{aligned} Step 3: Calculate the heat released by the combustion process. \begin{aligned} q_\text{ combustion process} &= -q_\text{ substance} \\ \\ &=-9790.98 \text{ J} \\ \\ &=-9.79098 \text{ kJ} \end{aligned} Step 4: Calculate the enthalpy change of the process. \begin{aligned} n(C_2H_5OH) & = \dfrac{n}{MM} \\ \\ &=\dfrac{250.35-249.84}{2 \times 12.01 + 6 \times 1.008 + 16} \\ \\ &=0.01107 \dots \text{ mol} \end{aligned}
\begin{aligned} \Delta H_c&=\dfrac{q_\text{ combustion process}}{n_\text{ combustion process}} \\ \\ & = -\dfrac{9.79098}{0.01107 \dots} \\ \\ & = -884.4134 \dots \\ \\ \therefore \Delta H_c& = - 884 \text{ kJ mol}^{-1} \text{(3 s.f.)} \end{aligned} Part b) If 35% of the heat produced was lost, then – 884.4134 … represents 65% of the molar enthalpy of combustion of ethanol. Therefore, the actual molar enthalpy of combustion is given by: \Delta H_c = \dfrac{-884.4134}{65} \times 100 = -1360 \text{ kJ mol}^{-1} \text{(3 s.f.)} |
| 2 | Step 1: Write a balanced chemical equation of the process. {C_4H_9OH}_{(l)} + 6{O_2}_{(g)} \rarr 5{H_2O}_{(l)} + 4{CO_2}_{(g)}
Step 2: Calculate the heat gained by the substances water and copper can. \begin{aligned} q_\text{ substance} & = mc\Delta T \\ \\ & = 0.250 \text{ kg} \times (4.18 \times 10^{-3} \text{J kg}^{-1}\text{K}^{-1}) \times (50-20 \text{ K} + mc\Delta T \\ \\ & = 0.0553 \text{ kg} \times (0.4 \times 10^{-3} \text{J kg}^{-1}\text{K}^{-1}) \times (50-20 \text{ K} \\ \\ &= 32013.6 \text{ J} \end{aligned}
Step 3: Calculate the heat released by the combustion process. \begin{aligned} q_\text{ combustion process} &= -q_\text{ substance} \\ \\ &=-32013.6 \text{ J} \\ \\ &=-32.0136 \text{ kJ} \end{aligned}
Step 4: Calculate the enthalpy change of the process. \begin{aligned} n(CH_3OH) & = \dfrac{n}{MM} \\ \\ &=\dfrac{53.8-51.3}{4 \times 12.01 + 10 \times 1.008 + 16} \\ \\ &=0.033729 \dots \text{ mol} \end{aligned}
\begin{aligned} \Delta H_c&=\dfrac{q_\text{ combustion process}}{n_\text{ combustion process}} \\ \\ & = -\dfrac{-32.0136}{0.033729 \dots} \\ \\ & = - 950 \text{ kJ mol}^{-1} \text{(2 s.f.)} \end{aligned} |
Learnable Education and www.learnable.education, 2019. Unauthorised use and/or duplications of this material without express and written permission from this site's author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Learnable Education and www.learnable.education with appropriate and specific direction to the original content.