How to Answer 'Compare' Chemistry Questions | HSC Chemistry Key Verb Series Part 2
Answering ‘compare’ long response questions
Students find ‘compare’ Chemistry questions tricky to answer. Most students struggle to identify the comparative features and provide irrelevant and non-comparative response.
‘How to answer ‘compare’ Chemistry questions’ is a comprehensive guide for students on how to develop a structured scientific response that is specific, relevant and comparative.
In this article, we’re going to discuss;
- Types of ‘compare’ Chemistry questions
- The process for answering ‘compare’ Chemistry questions
- Sample ‘compare’ long response questions and answers using the ToC Framework
Types of ‘compare’ long response questions in the HSC Chemistry Exam
Compare chemistry questions are perceived to be relatively easy to answer. But it’s also the question type students can lose marks easily without knowing.
There is always one or more ‘compare’ question in the HSC Chemistry Exam.
The main type of compare questions asked in HSC Chemistry exams involve qualitative comparisons.
Examples of ‘compare’ questions in Chemistry exams
Marks allocated for an ‘compare’ long response question can vary from 3 to 7 marks. Examples of ‘compare’ questions with different marks are listed below.
| Question | Marks |
| Question 1 (2016 HSC Q31 c) Methane and water vapour react to form carbon monoxide and hydrogen in a closed container as shown. CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g) ΔH = +206 kJ Compare the impact on the equilibrium system of a decrease in volume of the container to the impact of a decrease in temperature. Refer to the equilibrium constant in your answer. | 3 |
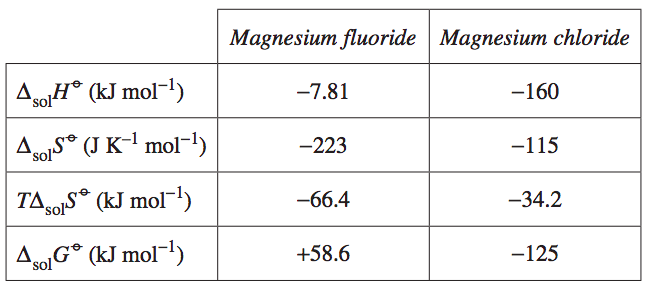
| Question 2 (2019 HSC Q30) The following data apply to magnesium fluoride and magnesium chloride dissolving in water at 298 K.
Compare the effects of enthalpy and entropy on the solubility of these salts. | 3 |
| Question 3 Compare the properties of acids and bases. | 4 |
| Question 4 Compare anionic, cationic and non-ionic synthetic detergents in terms of their use and chemical composition. | 5 |
| Question 5 Compare the use of infrared spectroscopy, 1H and 13C NMR spectroscopy in the identification of organic compounds. | 6 |
What is the process for answering ‘compare’ Chemistry questions?
To answer ‘compare’ chemistry questions, you must first understand the meaning of the verb, ‘compare’.
The NSW Education Standards Authority defines ‘compare’ as “show how things are similar or different.”
When answering ‘compare’ questions, students are required to show similarities and/or differences between two or more things.
Compare requires similarities and/or differences whereas ‘contrast’ requires differences only.
One of the ways to show similarities and/or differences using Learnable’s ToC FrameworkTM to construct a specific and relevant response.
Learnable’s ToC FrameworkTM
The ToC FrameworkTM or Table of Comparison helps you compare similarities and/or differences between two or more things in a structured manner and meet the marking criteria for ‘compare’ questions.
A ToC for comparing 2 objects is shown below.
| Features | Object 1 | Object 2 |
| Step 1: Identify a feature for comparison | Step 2a: Provide a qualitative or quantitative comparative detail based on feature identified. | Step 2b: Provide a qualitative or quantitative comparative detail based on feature identified. |
| Feature 2 | ||
| Feature 3 |
A ToC for comparing 3 objects is shown below.
| Object 1 | Object 2 | Object 3 | |
| Feature 1 | |||
| Feature 2 | |||
| Feature 3 |
When comparing 2 objects, you need to construct a 3 column ToC. When comparing 3 objects, you need to construct a 4 column ToC.
Sample ‘compare’ Chemistry questions and answers using the ToC FrameworkTM
Let’s apply Learnable’s ToC Framework to answer two ‘compare’ Chemistry questions.
Question 1 (3 marks): Module 5
Methane and water vapour react to form carbon monoxide and hydrogen in a closed container as shown.
CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g) ΔH = +206 kJ
Compare the impact on the equilibrium system of a decrease in volume of the container to the impact of a decrease in temperature. Refer to the equilibrium constant in your answer.
Solution to Question 1
Step 1: Identify the features that require a direct comparison.
| Feature | Decrease in volume of the container | Decrease in temperature |
| Disturbance to equilibrium | ||
| Shift in equilibrium | ||
| Effect on equilibrium constant |
Step 2: Provide a qualitative or quantitative comparative detail for each feature identified.
| Feature | Decrease in volume of the container | Decrease in temperature |
| Disturbance to equilibrium | Increase in pressure | Decrease in temperature |
| Shift in equilibrium | Equilibrium shifts left to the side with less moles of gas, to decrease pressure and minimise disturbance. | Equilibrium shifts left in the exothermic direction to replace heat and minimise disturbance. |
| Effect on equilibrium constant | Remains the same | Decreases |
Step 3: Construct a specific, relevant and comparative response.
A sample response to the question using the ToC Framework is shown below.
A decrease in volume of the container causes an increase in pressure which disturbs equilibrium. According to Le Chatelier’s principle, equilibrium shifts left to the side with less moles of gas, to decrease pressure and minimise disturbance. Similarly, a decrease in temperature disturbs equilibrium. According to Le Chatelier’s principle, equilibrium shifts left in the exothermic direction to replace heat and minimise disturbance. However, while a decrease in volume of the container has no effect on the equilibrium constant, a decrease in temperature decreases the value of the equilibrium constant.
Using keywords such as ‘similarly’ and ‘however’ can help highlight the similarities and differences you have identified in your response.
Question 2 (5 marks): Module 7
Compare anionic, cationic and non-ionic synthetic detergents in terms of their use and chemical composition.
Solution to Question 2
Step 1: Identify the features that require a direct comparison.
| Feature | Anionic detergents | Cationic detergents | Non-ionic detergents |
| Chemical composition | |||
| Properties | |||
| Use |
Step 2: Provide a qualitative or quantitative comparative detail for each feature identified.
| Feature | Anionic detergents | Cationic detergents | Non-ionic detergents |
| Chemical composition | 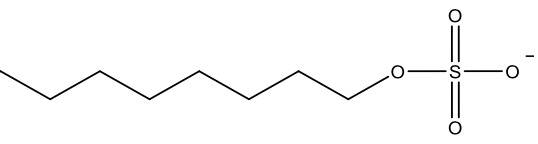 Anionic detergents have negatively charged polar heads, usually a sulfonate group and a long hydrocarbon tail. | 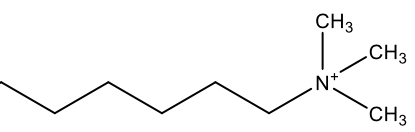 Cationic detergents have positively charged polar heads, usually a quaternary ammonium ion and a long hydrocarbon tail. | 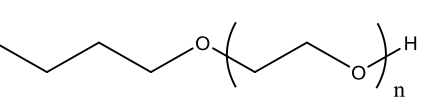 Non-ionic detergents have uncharged polar heads, such as ethoxylate units and a long hydrocarbon tail. |
| Properties | Do not form insoluble precipitates in hard water. Have a strong cleaning ability and are relatively inexpensive. | Do not form insoluble precipitates in hard water. Are strongly attracted to negatively charged surfaces including wet hair and fabric which acquire negative charges when wet. | Do not form insoluble precipitates in hard water. Do not foam as much as anionic and cationic detergents. |
| Use | Shampoos, dishwashing detergents and washing powders. | Fabric softeners and hair conditioners. | Dishwashing powder and washing powders in front loading washing machines. |
Step 3: Construct a specific, relevant and comparative response.
A comparison of the chemical composition, properties and uses of anionic, cationic and non-ionic synthetic detergents is given in the table below.
| Feature | Anionic detergents | Cationic detergents | Non-ionic detergents |
| Chemical composition |  Anionic detergents have negatively charged polar heads, usually a sulfonate group and a long hydrocarbon tail. |  Cationic detergents have positively charged polar heads, usually a quaternary ammonium ion and a long hydrocarbon tail. |  Non-ionic detergents have uncharged polar heads, such as ethoxylate units and a long hydrocarbon tail. |
| Properties | Do not form insoluble precipitates in hard water. Have a strong cleaning ability and are relatively inexpensive. | Do not form insoluble precipitates in hard water. Are strongly attracted to negatively charged surfaces including wet hair and fabric which acquire negative charges when wet. | Do not form insoluble precipitates in hard water. Do not foam as much as anionic and cationic detergents. |
| Use | Shampoos, dishwashing detergents and washing powders. | Fabric softeners and hair conditioners. | Dishwashing powder and washing powders in front loading washing machines. |
The table of comparison in Step 2 can be presented as the answer. In fact the HSC Marketing Committee recommends that students use a table for answering ‘compare’ Chemistry questions.
Learnable Education and www.learnable.education, 2019. Unauthorised use and/or duplications of this material without express and written permission from this site's author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Learnable Education and www.learnable.education with appropriate and specific direction to the original content.