How to Answer 'Explain' Chemistry Questions | HSC Chemistry Key Verb Series Part 1
Answering ‘Explain’ long-response Chemistry questions
Don’t know why or where you are losing marks in extended response questions? Most Year 11 and 12 Chemistry students find answering ‘explain’ Chemistry questions difficult and challenging.
‘How to answer ‘Explain’ Long Response Questions’ is a comprehensive guide for Year 12 students on how to develop logical, sequential and coherent answers to extended response questions requiring you to ‘Explain’.
In this article we discuss:
- Types of explain questions in the HSC Chemistry Exam
- The process for answering ‘Explain’ Chemistry questions
- Sample explain questions and answers using the CEO Framework
Types of explain questions in the HSC Chemistry Exam
‘Explain’ questions make up a significant proportion of your HSC Chemistry exam mark, and is critical to achieving a Band 6 result.
In the 2019 HSC Chemistry Exam, ‘Explain’ questions accounted for 23 marks. That’s 23% of the total exam mark!
Marks allocated for an ‘explain’ question can vary from 2 to 9 marks. Examples of explain questions with different marks are listed below.
| Question | Marks |
| Explain the relationship between the Ka of an acid and the conductivity of its aqueous solution. | 2 |
| Explain how ultraviolet visible spectroscopy is used to assist in the identification of organic compounds. | 3 |
| Explain why tertiary alcohols have lower boiling points than primary alcohols with the same molecular mass. | 3 |
| With reference to rate of reaction and yield, explain why the monitoring of both temperature and pressure is crucial for the Haber process. | 5 |
| Limestone (CaCO3) contributes to the hardness of water by releasing Ca2+ ions. The following chemical equation represents this reaction. CaCO3(s) + H2O(l) + CO2(g) ⇌ Ca2+(aq) + 2HCO3 −(aq) (ΔH < 0) It has been suggested that heating water reduces its hardness. Explain how this suggestion can be tested accurately, validly and reliably. | 9 |
What is the process for answering ‘Explain’ Chemistry questions?
To answer ‘explain’ chemistry questions, you must first understand the meaning of the verb, ‘explain.
The NSW Education Standards Authority defines ‘Explain’ as “relating cause and effect, making the relationship between things evident; provide why and/or how.”
When answering ‘explain’ questions, students are required to relate the cause and effect using scientific arguments. In addition, the response needs to be presented and structured in a logical and sequential manner.
One of the methods for answering ‘Explain’ questions is using Learnable’s CEO FrameworkTM (Cause, Effect, Outcome) to construct a logical and sequential response.
Learnable CEO FrameworkTM
The CEO Framework helps you structure your answer and meet the marking criteria for ‘explain’ questions. View the 3 step process below.
| Step | CEO Framework | Detail |
| 1 | Cause | Identify the cause |
| 2 | Effect | Describe the effect (due to the cause) by referring to the relevant law of Chemistry. |
| 3 | Outcome | State the outcome. |
When answering ‘Explain’ questions, you must include a reference to a relevant Law of Chemistry where possible.
Sample ‘explain’ Chemistry questions and answers using the CEO FrameworkTM
Let’s apply Learnable’s CEO Framework to answer the two types of ‘explain’ Chemistry questions.
- Explain why
- Explain how
Question 1 (2 Marks): Module 5
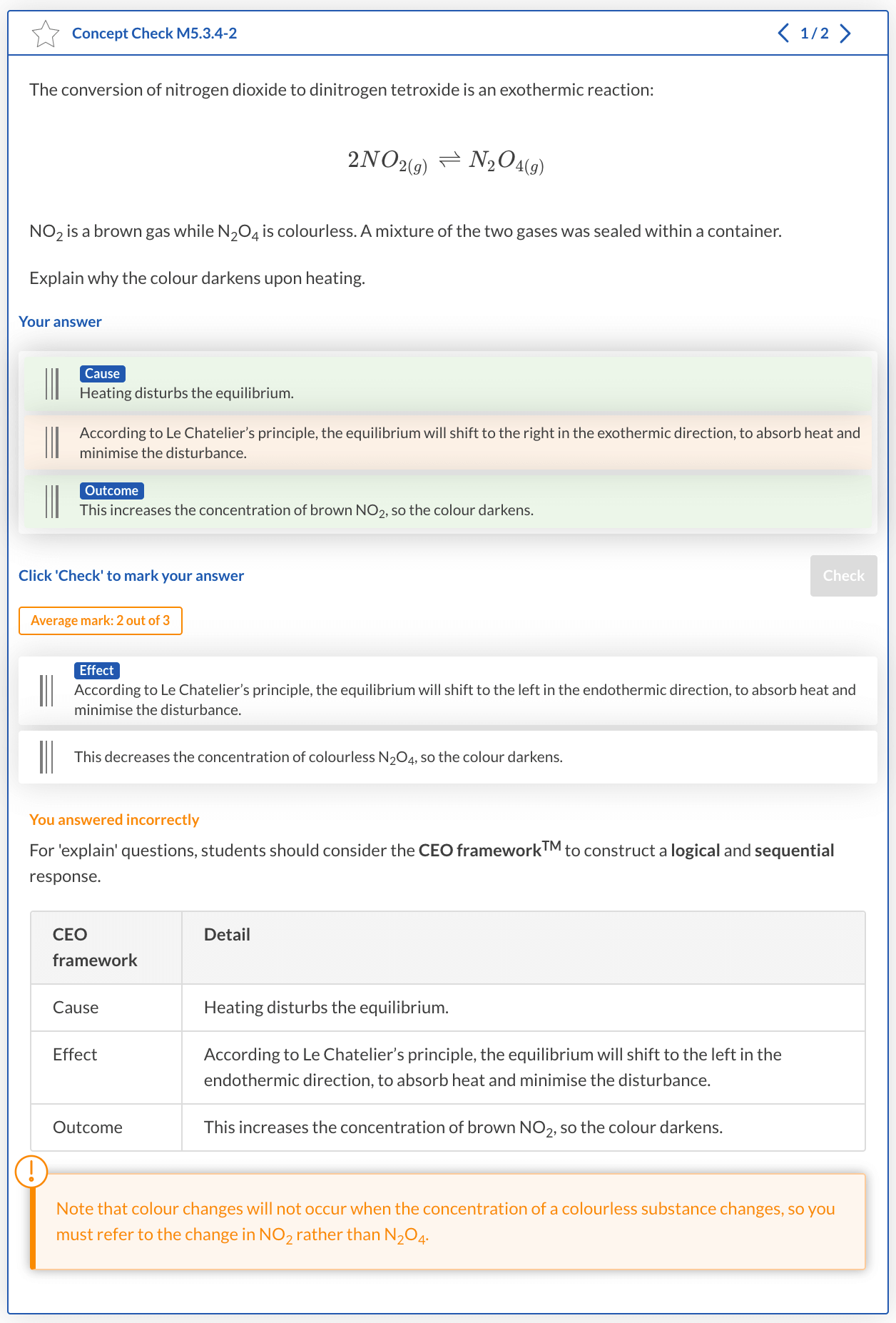
The conversion of nitrogen dioxide to dinitrogen tetroxide is an exothermic reaction.
2NO2(g) ⇌ N2O4(g)
NO2 is a brown gas while N2O4 is a colourless. A mixture of the two gases was sealed within a container.
Explain why the colour darkens upon heating?
Solution
Step 1: Identify the cause.
In this question, the cause must relate the action of heating the system to the equilibrium.
| Step | CEO Framework | Detail |
| 1 | Cause | Heating disturbs equilibrium. |
Step 2: Describe the effect due to the cause.
Our effect must derive the relationship between the change in reaction conditions and the corresponding shift in equilibrium using a scientific argument.
| Step | CEO Framework | Detail |
| 1 | Cause | Heating disturbs equilibrium. |
| 2 | Effect | According to Le Chatelier’s principle, the equilibrium will shift to the left in the endothermic direction, to absorb heat and minimise the disturbance. |
Step 3: State the outcome.
Our outcome must be a direct result of the effect outlined in Step 2.
| Step | CEO Framework | Detail |
| 1 | Cause | Heating disturbs equilibrium. |
| 2 | Effect | According to Le Chatelier’s principle, the equilibrium will shift to the left in the endothermic direction, to absorb heat and minimise the disturbance. |
| 3 | Outcome | This increases the concentration of brown NO2, so the colour darkens. |
Step 4: Construct a logical and sequential response.
A sample response to the question using the CEO FrameworkTM is shown below.
Heating disturbs equilibrium. According to Le Chatelier’s principle, the equilibrium will shift to the left in the endothermic direction, to absorb heat and minimise the disturbance. This increases the concentration of brown NO2, so the colour darkens.
Gain a competitive edge with Learnable’s interactive response templates
Learnable’s interactive response templates help you easily construct concise band 6 responses. Gain access to our library of long response questions and enhance your ability to develop a coherent scientific response.
Additional sample ‘explain’ Chemistry questions and answers using the CEO FrameworkTM
Question 2 (3 Marks): Module 7
Explain why tertiary alcohols have lower boiling points than primary alcohols with the same molecular mass.
Solution
Step 1: Identify the cause.
In this question, the cause must relate to the difference in structure and behaviour of primary and tertiary alcohols.
| Step | CEO Framework | Detail |
| 1 | Cause | Tertiary alcohols have an alkyl group adjacent to the hydroxyl functional group which prevents the hydroxyl groups from getting close together and restricts its ability to form strong hydrogen bonds. |
Step 2: Describe the effect due to the cause.
Our effect must derive the relationship between the structure/behaviour of the alcohol and the thermal energy required to separate the molecules. It must link these two points using a scientific argument.
| Step | CEO Framework | Detail |
| 1 | Cause | Tertiary alcohols have an alkyl group adjacent to the hydroxyl functional group which prevents the hydroxyl groups from getting close together and restricts its ability to form strong hydrogen bonds. |
| 2 | Effect | Therefore, the hydrogen bonds within tertiary alcohols are significantly weaker and less thermal energy is required to overcome the intermolecular forces of tertiary alcohols in comparison to primary alcohols of the same molecular mass. |
Step 3: State the outcome.
Our outcome must be a direct result of the effect outlined in Step 2.
| Step | CEO Framework | Detail |
| 1 | Cause | Tertiary alcohols have an alkyl group adjacent to the hydroxyl functional group which prevents the hydroxyl groups from getting close together and restricts its ability to form strong hydrogen bonds. |
| 2 | Effect | Therefore, the hydrogen bonds within tertiary alcohols are significantly weaker and less thermal energy is required to overcome the intermolecular forces of tertiary alcohols in comparison to primary alcohols of the same molecular mass. |
| 3 | Outcome | Hence, tertiary alcohols have lower boiling points than primary alcohols. |
Step 4: Construct a logical and sequential response.
A sample response to the question using the CEO FrameworkTM is shown below.
Tertiary alcohols have an alkyl group adjacent to the hydroxyl functional group which prevents the hydroxyl groups from getting close together and restricts its ability to form strong hydrogen bonds. Therefore, the hydrogen bonds within tertiary alcohols are significantly weaker and less thermal energy is required to overcome the intermolecular forces of tertiary alcohols in comparison to primary alcohols. Hence, tertiary alcohols have lower boiling points than primary alcohols.
The sequential links between cause, effect and outcome are bolded in our sample answer. This ensures our answer features a logical argument from start to finish.
Question 3 (3 Marks): Module 8
Explain how ultraviolet visible spectroscopy is used to assist in the identification of organic compounds.
Solution
In this question, students are asked to relate the principle behind ultraviolet visible spectroscopy (cause) to its ability to identify organic compounds (effect).
| Step | CEO Framework | Detail |
| 1 | Cause | The absorption of UV and/or visible radiation by a molecule leads to the transition of an electron from a lower energy molecular orbital to a higher energy molecular orbital. The wavelength absorbed corresponds to the energy gap between molecular levels. With increasing conjugation, the energy gap becomes smaller. |
| 2 | Effect | Therefore, the energy gap is dependent on the type of bonding and extent of conjugation in the molecule. |
| 3 | Outcome | Hence, by measuring the wavelengths absorbed by a sample and comparing the λmax with a database of standards, the type of bonding and extent of conjugation in an organic compound can be identified. |
A sample response to the question using the CEO FrameworkTM is shown below.
The absorption of UV and/or visible radiation by a molecule leads to the transition of an electron from a lower energy molecular orbital to a higher energy molecular orbital. The wavelength absorbed corresponds to the energy gap between molecular levels. With increasing conjugation, the energy gap becomes smaller. Therefore, the energy gap is dependent on the type of bonding and extent of conjugation in the molecule. Hence, by measuring the wavelengths absorbed by a sample and comparing the λmax with a database of standards, the type of bonding and extent of conjugation in an organic compound can be identified.
Get free access to Band 6 long response templates that help you structure your answer easily.
Take the guesswork out. Learnable’s Band 6 response templates help you answer explain, discuss, assess questions easily. Join 10000+ students who are getting ahead with learnable. Try for free now.
Learnable Education and www.learnable.education, 2019. Unauthorised use and/or duplications of this material without express and written permission from this site's author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Learnable Education and www.learnable.education with appropriate and specific direction to the original content.